We serve Chemical Name:2-Methyl-6-(trifluoromethyl)nicotinaldehyde CAS:545394-83-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
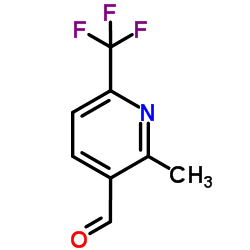
Chemical Name:2-Methyl-6-(trifluoromethyl)nicotinaldehyde
CAS.NO:545394-83-0
Synonyms:2-methyl-6-(trifluoromethyl)pyridine-3-carbaldehyde;3-Pyridinecarboxaldehyde, 2-methyl-6-(trifluoromethyl)-;2-Methyl-6-(trifluoromethyl)nicotinaldehyde
Molecular Formula:C8H6F3NO
Molecular Weight:189.135
HS Code:
Physical and Chemical Properties:
Melting point:157-161ºC
Boiling point:209.7±40.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.480
PSA:29.96000
Exact Mass:189.040146
LogP:2.40
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-methyl-6-(trifluoromethyl)pyridine-3-carbaldehyde chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Methyl-6-(trifluoromethyl)nicotinaldehyde physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Methyl-6-(trifluoromethyl)nicotinaldehyde Use and application,2-methyl-6-(trifluoromethyl)pyridine-3-carbaldehyde technical grade,usp/ep/jp grade.
Related News: The risk-based score generated by the tool helps sponsors gain advance understanding of the appropriate level of clinical supply management oversight needed to help get their project started on time and maintain momentum as the study progresses. 2-Methyl-6-(trifluoromethyl)nicotinaldehyde manufacturer he agency said the drugmaker and Emergent must agree that the FDA can share relevant information about the manufacturing of the doses with regulators where the vaccine is shipped. 2-Methyl-6-(trifluoromethyl)nicotinaldehyde supplier As the first-to-market BTK inhibitor, Imbruvica has been treating front-line CLL patients since its monotherapy go-ahead in early 2016. But it’s now facing competition from AstraZeneca’s Calquence, which just detailed a head-to-head safety advantage over Imbruvica in previously treated CLL. 2-Methyl-6-(trifluoromethyl)nicotinaldehyde vendor The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency. 2-Methyl-6-(trifluoromethyl)nicotinaldehyde factory he agency said the drugmaker and Emergent must agree that the FDA can share relevant information about the manufacturing of the doses with regulators where the vaccine is shipped.

