We serve Chemical Name:tyrphostin a63 CAS:5553-97-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
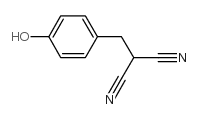
Chemical Name:tyrphostin a63
CAS.NO:5553-97-9
Synonyms:MFCD00133904;(4-hydroxybenzyl)propanedinitrile;(4-Hydroxy-benzyl)-malononitril;benzylidenemalononitrile (BMN) deriv. 63;Tyrphostin A63;2-(4-hydroxybenzyl)malononitrile;tyrphostin 63;4-hydroxybenzylmalononitrile;AG 43
Molecular Formula:C10H8N2O
Molecular Weight:172.18300
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:421ºC at 760 mmHg
Density:1.222g/cm3
Index of Refraction:1.577
PSA:67.81000
Exact Mass:172.06400
LogP:1.59806
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like MFCD00133904 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,AG 43 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,benzylidenemalononitrile (BMN) deriv. 63 Use and application,AG 43 technical grade,usp/ep/jp grade.
Related News: DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation. tyrphostin a63 manufacturer According to statistics from the Price Supervision and Competition Bureau of the National Development and Reform Commission, China can produce more than 1,500 kinds of bulk drugs, with a total output of one million tons and an export volume of more than 60%. It has become the world’s second largest drug substance after the United States. Country of manufacture and largest exporter. tyrphostin a63 supplier Beta Bionics is committed to obtaining regulatory approval and commercializing all three iLet configurations. tyrphostin a63 vendor DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation. tyrphostin a63 factory DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.

