We serve Chemical Name:sanguinarium chloride CAS:5578-73-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
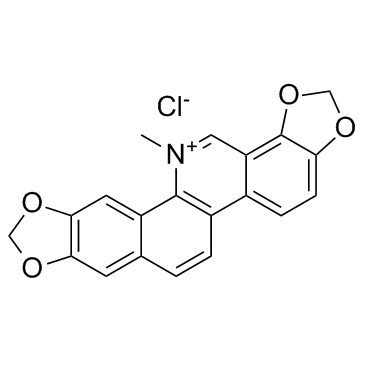
Chemical Name:sanguinarium chloride
CAS.NO:5578-73-4
Synonyms:Sanguinarine chloride;Sanguinarine chloride hydrate;13-methyl[1,3]benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridin-13-ium chloride;13-methyl[1,3]benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridinium chloride;13-Methyl[1,3]benzodioxolo[5,6-c][1,3]dioxolo[4,5-i]phenanthridin-13-ium chloride;[1,3]Benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridinium, 13-methyl-, chloride (1:1);sanguinarine;13-methyl[1,3]benzodioxolo[5,6-c]-1,3-dioxolo[4,5-i]phenanthridinium chloride (1:1);chlorure de sanguinarium;sanguinarium chloride;13-Methyl[1,3]dioxolo[4,5]benzo[1,2-c][1,3]dioxolo[4,5-i]phenanthridin-13-iumchlorid;Sanguinarine (chloride)
Molecular Formula:C20H14ClNO4
Molecular Weight:367.783
HS Code:
Physical and Chemical Properties:
Melting point:287-289 ºC
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:40.80000
Exact Mass:367.061127
LogP:0.43210
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Sanguinarine chloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Sanguinarine (chloride) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Sanguinarine chloride Use and application,13-Methyl[1,3]dioxolo[4,5]benzo[1,2-c][1,3]dioxolo[4,5-i]phenanthridin-13-iumchlorid technical grade,usp/ep/jp grade.
Related News: Most of California’s COVID-19 restrictions were lifted on Tuesday and the nation’s most populous state is poised to come roaring back,” according to Gov. Gavin Newsom.
State social distancing rules and capacity limits at restaurants, bars, supermarkets, gyms, stadiums and other locations have been rolled back, and masks will no longer be required for vaccinated people in most settings, the Associated Press reported.
However, businesses and counties can still require them.
In March 2020, California became the first in the United States to impose a statewide shutdown. More people tested positive for the virus (3.8 million to date) and more people died (more than 63,000) in California than anywhere else in the country, but it had a lower per capita death rate than most others, the AP reported.
California now has one of the lowest infection rates (below 1%) in the U.S.
That low rate and a rising number of vaccinated residents — over 70% of adults have had at least one shot — led Newsom to announce in April that most pandemic restrictions would be lifted June 15, the AP reported.
However, the governor has repeatedly said the reopening doesn’t mean the pandemic is over, and some public health measures will still be in place for “mega events. sanguinarium chloride manufacturer Factors that have a significant impact on the sales volume of APIs come from the production side, which mainly includes costs and processes. sanguinarium chloride supplier The Company��s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs). sanguinarium chloride vendor Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance. sanguinarium chloride factory The Company��s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs).
State social distancing rules and capacity limits at restaurants, bars, supermarkets, gyms, stadiums and other locations have been rolled back, and masks will no longer be required for vaccinated people in most settings, the Associated Press reported.
However, businesses and counties can still require them.
In March 2020, California became the first in the United States to impose a statewide shutdown. More people tested positive for the virus (3.8 million to date) and more people died (more than 63,000) in California than anywhere else in the country, but it had a lower per capita death rate than most others, the AP reported.
California now has one of the lowest infection rates (below 1%) in the U.S.
That low rate and a rising number of vaccinated residents — over 70% of adults have had at least one shot — led Newsom to announce in April that most pandemic restrictions would be lifted June 15, the AP reported.
However, the governor has repeatedly said the reopening doesn’t mean the pandemic is over, and some public health measures will still be in place for “mega events. sanguinarium chloride manufacturer Factors that have a significant impact on the sales volume of APIs come from the production side, which mainly includes costs and processes. sanguinarium chloride supplier The Company��s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs). sanguinarium chloride vendor Exceeding impurities in the drug substance may cause the product and its preparation to be recalled. The company receives an FDA warning letter or a CEP certificate suspension, which in turn will cause customer compensation, product recall costs, and asset impairment losses to affect the company’s performance. sanguinarium chloride factory The Company��s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs).

