We serve Chemical Name:tris(2,2,3,3,4,4,4-heptafluorobutyl) phosphate CAS:563-09-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
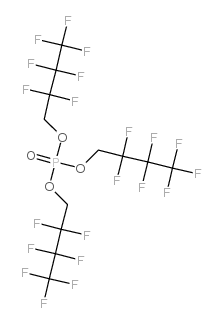
Chemical Name:tris(2,2,3,3,4,4,4-heptafluorobutyl) phosphate
CAS.NO:563-09-7
Synonyms:pc6959
Molecular Formula:C12H6F21O4P
Molecular Weight:644.11400
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:268.4ºC at 760 mmHg
Density:1.674g/cm3
Index of Refraction:1.304
PSA:54.57000
Exact Mass:643.96700
LogP:7.64300
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like pc6959 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,pc6959 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,pc6959 Use and application,pc6959 technical grade,usp/ep/jp grade.
Related News: The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented. tris(2,2,3,3,4,4,4-heptafluorobutyl) phosphate manufacturer The entire set of results “strongly support” the Darzalex-Rd combo as a new standard of care for newly diagnosed, transplant-ineligible multiple myeloma patients, Thierry Facon, M.D., an investigator of the MAIA trial, said in a statement. tris(2,2,3,3,4,4,4-heptafluorobutyl) phosphate supplier The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population. tris(2,2,3,3,4,4,4-heptafluorobutyl) phosphate vendor In March 2021, Reuters reported that a former Lilly human resources officer, Amrit Mula, had identified internally some of the same violations later documented by the FDA. Mula was forced out of the company in early 2019 after Lilly executives sought to downplay her findings, according to a letter demanding compensation for damages that her attorneys sent to the company. tris(2,2,3,3,4,4,4-heptafluorobutyl) phosphate factory The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population.

