We serve Chemical Name:DMBA CAS:57-97-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
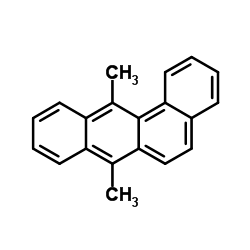
Chemical Name:DMBA
CAS.NO:57-97-6
Synonyms:EINECS 200-359-5;DMBA;7,12-Dimethyltetraphene;MFCD00003600;7,12-Dimethylbenzo[a]anthracene;Benz[a]anthracene, 7,12-dimethyl-;7,12-Dimethylbenz[a]anthracene
Molecular Formula:C20H16
Molecular Weight:256.341
HS Code:
Physical and Chemical Properties:
Melting point:122-123 °C(lit.)
Boiling point:463.5±15.0 °C at 760 mmHg
Density:1.1±0.1 g/cm3
Index of Refraction:1.729
PSA:
Exact Mass:256.125214
LogP:6.83
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 3077 9/PG 3
Packing Group:III
Contact us for information like EINECS 200-359-5 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,7,12-Dimethylbenz[a]anthracene physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,7,12-Dimethyltetraphene Use and application,MFCD00003600 technical grade,usp/ep/jp grade.
Related News: The overall competition in China’s specialty drug substance market is relatively moderate, but due to the higher technical barriers and higher entry barriers for new entrants, there will be no obvious intensified competition in the short term. DMBA manufacturer The program will run alongside our ongoing Phase 3 INSPIRE Trial, and is expected to continue until commercial launch in such countries. DMBA supplier Analysts at Mizuho Americas, who spoke to Lilly’s management this week, said that may well have now changed. “Overall, it sounds like the approval raises new questions for Lilly (as it does for many of us!),” the firm said in a note to clients. DMBA vendor Our pharma and biotech offering includes registration and commercialization of products through in-licensing and flexible partnerships. DMBA factory Analysts at Mizuho Americas, who spoke to Lilly’s management this week, said that may well have now changed. “Overall, it sounds like the approval raises new questions for Lilly (as it does for many of us!),” the firm said in a note to clients.

