We serve Chemical Name:(Z)-2-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-enal CAS:58102-02-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
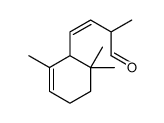
Chemical Name:(Z)-2-methyl-4-(2,6,6-trimethylcyclohex-2-en-1-yl)but-3-enal
CAS.NO:58102-02-6
Synonyms:optically inactive;EINECS 261-121-4
Molecular Formula:C14H22O
Molecular Weight:206.32400
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:275.4ºC at 760mmHg
Density:0.926g/cm3
Index of Refraction:1.508
PSA:17.07000
Exact Mass:206.16700
LogP:3.76010
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like optically inactive chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,EINECS 261-121-4 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,EINECS 261-121-4 Use and application,EINECS 261-121-4 technical grade,usp/ep/jp grade.
Related News: Soliris, first approved for generalized myasthenia gravis (gMG) in 2017, carries a list price of about $470,000 annually. But to ICER, that cost is “well beyond typical thresholds.” 6-amino-5-methyl-4-oxo-4,5-dihydro-[1,2,3]thiadiazolo[4,5-c]pyridine-7-carboxamide manufacturers During an investor event last month, Walmsley laid out her blueprint for the new GSK. It features a 5% average sales growth rate in the next five years, leading to total sales of £33 billion ($46 billion) by 2031. ((S)-2-((S)-4-Benzyl-2-oxo-oxazolidin-3-yl)-1-{(1R,2R)-2-[(S)-2-((S)-4-benzyl-2-oxo-oxazolidin-3-yl)-1-tert-butoxycarbonylamino-2-oxo-ethyl]-cyclopropyl}-2-oxo-ethyl)-carbamic acid tert-butyl ester suppliers The results provided a formulation amenable to two dosage strengths by applying a proportional dosing weight method.” The product is currently in late-stage development with the New Drug Application filing expected in late 2021. {(S)-2-[(2S,3S)-2-((S)-2-tert-Butoxycarbonylamino-propionylamino)-3-methyl-pentanoylamino]-propionylamino}-acetic acid 2,2,8-trimethyl-4H-[1,3]dioxino[4,5-c]pyridin-5-ylmethyl ester vendor & factory.

