We serve Chemical Name:2-Fluoro-4-methoxyaniline CAS:458-52-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
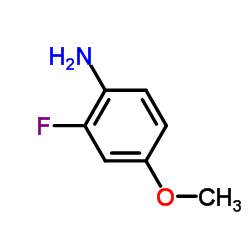
Chemical Name:2-Fluoro-4-methoxyaniline
CAS.NO:458-52-6
Synonyms:2-Fluoro-4-methoxybenzenamine;ZR BF DO1;4-Amino-3-fluoroanisole;2-Fluor-4-methoxy-anilin;2-fluoro-4-methoxy-aniline;Benzenamine, 2-fluoro-4-methoxy-;2-Fluoro-4-methoxy-phenylamine;2-Fluoro-4-methoxyaniline;2-Fluoro-p-anisidine;2-Fluor-p-anisidin
Molecular Formula:C7H8FNO
Molecular Weight:141.143
HS Code:2922299090
Physical and Chemical Properties:
Melting point:175ºC
Boiling point:203.0±20.0 °C at 760 mmHg
Density:1.2±0.1 g/cm3
Index of Refraction:1.532
PSA:35.25000
Exact Mass:141.058990
LogP:1.13
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-Fluoro-4-methoxybenzenamine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Fluor-p-anisidin physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,ZR BF DO1 Use and application,2-Fluor-p-anisidin technical grade,usp/ep/jp grade.
Related News: Factors that have a significant impact on the sales volume of APIs come from the production side, which mainly includes costs and processes. 2-Fluoro-4-methoxyaniline manufacturer Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country. 2-Fluoro-4-methoxyaniline supplier Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country. 2-Fluoro-4-methoxyaniline vendor Compared with existing Imbruvica therapies, where patients take the BTK inhibitor daily—potentially for years—until their disease progresses, the new Venclexta combo caps treatment at about 14 months. That could offer a new option for “probably younger patients with CLL that prefer more flexible, treatment-free intervals,” said Craig Tendler, M.D., vice president of oncology clinical development and global medical affairs at J&J’s Janssen unit, during an interview. 2-Fluoro-4-methoxyaniline factory Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

