We serve Chemical Name:2-(cyclohexen-1-yl)butanamide CAS:59-13-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
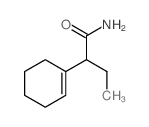
Chemical Name:2-(cyclohexen-1-yl)butanamide
CAS.NO:59-13-2
Synonyms:2-Cyclohex-1-enyl-buttersaeure-amid;2-cyclohex-1-enyl-butyric acid amide
Molecular Formula:C10H17NO
Molecular Weight:167.24800
HS Code:2924299090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:298.5ºC at 760 mmHg
Density:1g/cm3
Index of Refraction:1.502
PSA:43.09000
Exact Mass:167.13100
LogP:2.69860
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-Cyclohex-1-enyl-buttersaeure-amid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-cyclohex-1-enyl-butyric acid amide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-cyclohex-1-enyl-butyric acid amide Use and application,2-Cyclohex-1-enyl-buttersaeure-amid technical grade,usp/ep/jp grade.
Related News: Subsequently, the company launched the drug under the brand names ‘Remo’ and ‘Remozen’. 2-(cyclohexen-1-yl)butanamide manufacturer The insulin-only and bihormonal configurations may be helpful in diabetes. 2-(cyclohexen-1-yl)butanamide supplier He said the FDA made its decision by fiat, and had not asked its advisors to consider whether the drug’s ability to remove a type of brain plaques known as beta amyloid would improve outcomes for patients. 2-(cyclohexen-1-yl)butanamide vendor The insulin-only and bihormonal configurations may be helpful in diabetes. 2-(cyclohexen-1-yl)butanamide factory He said the FDA made its decision by fiat, and had not asked its advisors to consider whether the drug’s ability to remove a type of brain plaques known as beta amyloid would improve outcomes for patients.

