We serve Chemical Name:triethylarsane CAS:617-75-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
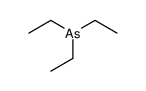
Chemical Name:triethylarsane
CAS.NO:617-75-4
Synonyms:triethyl arsine;Triaethyl-arsin;EINECS 210-526-4;Triethylarsenic;Arsine triethyl
Molecular Formula:C6H15As
Molecular Weight:162.10500
HS Code:
Physical and Chemical Properties:
Melting point:-91ºC
Boiling point:140ºC
Density:1.152 g/cm3
Index of Refraction:
PSA:
Exact Mass:162.03900
LogP:2.54090
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 3280
Packing Group:
Contact us for information like triethyl arsine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Arsine triethyl physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Arsine triethyl Use and application,Triaethyl-arsin technical grade,usp/ep/jp grade.
Related News: When they do design their phase 3, they’re going to need to be better about that part of the study design, and really focus on enrolling individuals that have the right biomarkers to be able to look at not only the safety and tolerability but also the efficacy on cognition and function,” Edelmayer said.
The vaccine approach is promising because using the body’s immune system to fight Alzheimer’s would sidestep one of the problems in developing a drug to treat the disease — namely that it’s difficult to design medications that can easily enter the brain and attack a specific target, Edelmayer explained.
Through booster doses given every three months, the vaccine is “training your body over time how to react to the pathological tau,” Edelmayer said. “You will likely need additional shots of this particular therapeutic to keep it as productive as possible in targeting that tau. triethylarsane manufacturer AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment. triethylarsane supplier AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment. triethylarsane vendor The memo denies the company made false statements to the FDA. In their complaint, employees said they were broadly concerned that quality control documents the FDA requires companies to maintain had been rewritten or fabricated. The employees did not specify whether these materials had been shown to the FDA. triethylarsane factory Darzalex has its own VRd combo trials, dubbed PERSEUS and CEPHEUS, which are testing a newly approved under-the-skin version of the J&J drug called Darzalex Faspro. The CEPHEUS trial appears to have just completed its primary analysis, according to a listing on ClinicalTrials.gov.
The vaccine approach is promising because using the body’s immune system to fight Alzheimer’s would sidestep one of the problems in developing a drug to treat the disease — namely that it’s difficult to design medications that can easily enter the brain and attack a specific target, Edelmayer explained.
Through booster doses given every three months, the vaccine is “training your body over time how to react to the pathological tau,” Edelmayer said. “You will likely need additional shots of this particular therapeutic to keep it as productive as possible in targeting that tau. triethylarsane manufacturer AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment. triethylarsane supplier AbbVie and Johnson & Johnson already boast three FDA-approved regimens for Imbruvica in newly diagnosed chronic lymphocytic leukemia (CLL). Now, the pair aims to add another to the drug’s label, one that could shorten the time patients need to stay on treatment. triethylarsane vendor The memo denies the company made false statements to the FDA. In their complaint, employees said they were broadly concerned that quality control documents the FDA requires companies to maintain had been rewritten or fabricated. The employees did not specify whether these materials had been shown to the FDA. triethylarsane factory Darzalex has its own VRd combo trials, dubbed PERSEUS and CEPHEUS, which are testing a newly approved under-the-skin version of the J&J drug called Darzalex Faspro. The CEPHEUS trial appears to have just completed its primary analysis, according to a listing on ClinicalTrials.gov.

