We serve Chemical Name:N,1-dimethylindole-2-carboxamide CAS:61939-18-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
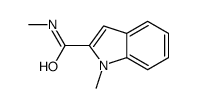
Chemical Name:N,1-dimethylindole-2-carboxamide
CAS.NO:61939-18-2
Synonyms:N,1-dimethyl-1H-indole-2-carboxamide;1-Methyl-indol-2-carbonsaeure-methylamid;1-methyl-2-(methylaminocarbonyl)indole;1H-Indole-2-carboxamide,N,1-dimethyl;1-methyl-indole-2-carboxylic acid methylamide;1-Methyl-indol-carbonsaeure-(2)-methylamid;N-methyl-2-(1-methylindole)carboxamide
Molecular Formula:C11H12N2O
Molecular Weight:188.22600
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:37.52000
Exact Mass:188.09500
LogP:2.11270
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like N,1-dimethyl-1H-indole-2-carboxamide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,N-methyl-2-(1-methylindole)carboxamide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1-Methyl-indol-2-carbonsaeure-methylamid Use and application,1-methyl-indole-2-carboxylic acid methylamide technical grade,usp/ep/jp grade.
Related News: FT was defined as any difficulty paying medical bills, high financial distress, cost-related medication nonadherence, food insecurity, and/or foregone/delayed care due to cost. N,1-dimethylindole-2-carboxamide manufacturer The survival benefit is “unprecedented” for an aging myeloma population, almost all of whom were 65 years of age or older, said Craig Tendler, M.D., vice president of oncology clinical development and global medical affairs at J&J’s Janssen unit, during an interview. N,1-dimethylindole-2-carboxamide supplier Analysts at Jefferies, meanwhile, said the FDA accelerated approval for Aduhelm, which was based on a surrogate endpoint of amyloid beta plaque reduction (and not clinical benefit) “has implications for ongoing AD studies,” most notably, it reckons, for Roche’s phase 3 GRADUATE test for its anti-amyloid-beta candidate gantenerumab, “as a much lower hurdle than demonstrating clear cognitive benefit” has now become precedent. Jefferies, still cautious, said it remains “unclear what stance FDA may take for the field if GRADUATE fails on cognition despite significant Abeta reductions,” but says it’s probably not going to revive Roche-AC Immune’s anti-amyloid-beta candidate crenezumab, which saw its late-stage CREAD trials discontinued for futility but which is currently in an Alzheimer’s prevention study. N,1-dimethylindole-2-carboxamide vendor ��With early evidence of clinical activity for our off-the-shelf, iPSC-derived NK cell programs, we are excited to lead in bringing next-generation CAR T-cell therapies to patients and plan to submit an IND for FT819 in the first half of 2020.�� N,1-dimethylindole-2-carboxamide factory The survival benefit is “unprecedented” for an aging myeloma population, almost all of whom were 65 years of age or older, said Craig Tendler, M.D., vice president of oncology clinical development and global medical affairs at J&J’s Janssen unit, during an interview.

