We serve Chemical Name:Ki20227 CAS:623142-96-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
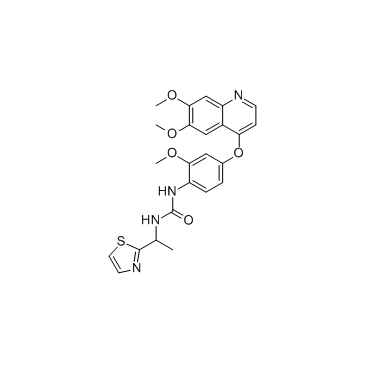
Chemical Name:Ki20227
CAS.NO:623142-96-1
Synonyms:1-{4-[(6,7-Dimethoxy-4-quinolinyl)oxy]-2-methoxyphenyl}-3-[1-(1,3-thiazol-2-yl)ethyl]urea;hms3244k11;hms3244l11;hms3244k12;Urea, N-[4-[(6,7-dimethoxy-4-quinolinyl)oxy]-2-methoxyphenyl]-N’-[1-(2-thiazolyl)ethyl]-;N-(4-((6,7-dimethoxy-4-quinolyl)oxy)-2-methoxyphenyl)-N’-(1-(1,3-thiazole-2-yl)ethyl)urea;Ki20227
Molecular Formula:C24H24N4O5S
Molecular Weight:480.536
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:621.8±55.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.651
PSA:132.07000
Exact Mass:480.146729
LogP:4.18
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1-{4-[(6,7-Dimethoxy-4-quinolinyl)oxy]-2-methoxyphenyl}-3-[1-(1,3-thiazol-2-yl)ethyl]urea chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Ki20227 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,hms3244k11 Use and application,hms3244k12 technical grade,usp/ep/jp grade.
Related News: The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy. Ki20227 manufacturer The GLOW trial doesn’t yet have the answer to that question, but Tendler pointed to encouraging early evidence from the regimen’s phase 2 trial, dubbed CAPTIVATE. Among eight patients who progressed after stopping the fixed-duration combo, six responded to subsequent Imbruvica monotherapy, while the other two didn’t have a response report, according to data presented at the recent American Society of Clinical Oncology annual meeting. Ki20227 supplier China has granted conditional approval to its first self-developed treatment for Alzheimer��s disease, a move that may point to revived opportunities in a therapeutic area where drugmakers have burned billions of dollars without yielding a validated new drug. Ki20227 vendor The company reported that it was cooperating with the Justice Department probe and that an outside law firm was also “investigating these allegations thoroughly.” Ki20227 factory The GLOW trial doesn’t yet have the answer to that question, but Tendler pointed to encouraging early evidence from the regimen’s phase 2 trial, dubbed CAPTIVATE. Among eight patients who progressed after stopping the fixed-duration combo, six responded to subsequent Imbruvica monotherapy, while the other two didn’t have a response report, according to data presented at the recent American Society of Clinical Oncology annual meeting.

