We serve Chemical Name:4-Methyl-N-(3-phenylpropyl)aniline CAS:63980-34-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
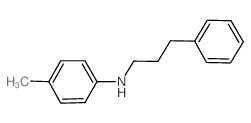
Chemical Name:4-Methyl-N-(3-phenylpropyl)aniline
CAS.NO:63980-34-7
Synonyms:Benzenamine,4-methyl-N-(phenylpropyl);p-Toluidine,N-phenylpropyl;N-Phenylpropyl-p-toluidine
Molecular Formula:C16H19N
Molecular Weight:225.32900
HS Code:2921430090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:375.2ºC at 760mmHg
Density:1.029g/cm3
Index of Refraction:1.595
PSA:12.03000
Exact Mass:225.15200
LogP:4.11270
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Benzenamine,4-methyl-N-(phenylpropyl) chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,N-Phenylpropyl-p-toluidine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Benzenamine,4-methyl-N-(phenylpropyl) Use and application,N-Phenylpropyl-p-toluidine technical grade,usp/ep/jp grade.
Related News: A new case of coronavirus has been reported in the United Arab Emirates, the country’s fifth, the Ministry of Health and Prevention announced on Saturday. 4-Methyl-N-(3-phenylpropyl)aniline manufacturer Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country. 4-Methyl-N-(3-phenylpropyl)aniline supplier Onconova has conducted trials with two other research compounds and has a pre-clinical program with a CDK4/6 and Ark5 inhibitor, ON 123300. 4-Methyl-N-(3-phenylpropyl)aniline vendor The quality of the drug substance determines the quality of the preparation, so its quality standards are very strict. Countries around the world have formulated strict national pharmacopoeia standards and quality control methods for their widely used drug substances. 4-Methyl-N-(3-phenylpropyl)aniline factory Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

