We serve Chemical Name:Glafenine Hydrochloride CAS:65513-72-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
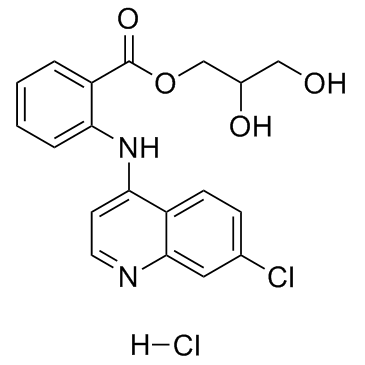
Chemical Name:Glafenine Hydrochloride
CAS.NO:65513-72-6
Synonyms:Glafenine hydrochloride;Prestwick_628;Glafenine (hydrochloride)
Molecular Formula:C19H18Cl2N2O4
Molecular Weight:409.26300
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:618ºC at 760 mmHg
Density:N/A
Index of Refraction:
PSA:91.68000
Exact Mass:408.06400
LogP:4.01680
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like Glafenine hydrochloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Glafenine (hydrochloride) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Glafenine (hydrochloride) Use and application,Glafenine hydrochloride technical grade,usp/ep/jp grade.
Related News: With the chemical industry being one of the most polluting, global efforts are heavily focused on developing a more sustainable and greener footprint. phosphanyloxyphosphane manufacturers The Institute for Clinical and Economic Review (ICER) on Friday released its report examining the cost-effectiveness of Soliris, also known as eculizumab, as well as up-and-comer efgartigimod for patients with the myasthenia gravis. Sodium Azide-15N3 suppliers With the chemical industry being one of the most polluting, global efforts are heavily focused on developing a more sustainable and greener footprint. azanium,silver,sulfate vendor & factory Today’s announcement is strongly aligned to IDA Ireland’s Regional pillar and its continued commitment to winning jobs and investment in regional locations. I wish Charles River every success with this expansion.”,The Institute for Clinical and Economic Review (ICER) on Friday released its report examining the cost-effectiveness of Soliris, also known as eculizumab, as well as up-and-comer efgartigimod for patients with the myasthenia gravis.

