We serve Chemical Name:2,6-pipecolinoxylidide hydrochloride CAS:65797-42-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
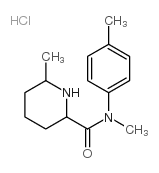
Chemical Name:2,6-pipecolinoxylidide hydrochloride
CAS.NO:65797-42-4
Synonyms:2,6-pipecolinoxylidide hydrochloride
Molecular Formula:C15H23ClN2O
Molecular Weight:282.80900
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:32.34000
Exact Mass:282.15000
LogP:3.61920
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2,6-pipecolinoxylidide hydrochloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,6-pipecolinoxylidide hydrochloride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,6-pipecolinoxylidide hydrochloride Use and application,2,6-pipecolinoxylidide hydrochloride technical grade,usp/ep/jp grade.
Related News: As the first-to-market BTK inhibitor, Imbruvica has been treating front-line CLL patients since its monotherapy go-ahead in early 2016. But it’s now facing competition from AstraZeneca’s Calquence, which just detailed a head-to-head safety advantage over Imbruvica in previously treated CLL. 2,6-pipecolinoxylidide hydrochloride manufacturer It does not require a production license for the drug substance, and can be produced in an ordinary chemical plant. As long as it reaches a certain level, it can be used for the synthesis of the drug substance. 2,6-pipecolinoxylidide hydrochloride supplier As the first-to-market BTK inhibitor, Imbruvica has been treating front-line CLL patients since its monotherapy go-ahead in early 2016. But it’s now facing competition from AstraZeneca’s Calquence, which just detailed a head-to-head safety advantage over Imbruvica in previously treated CLL. 2,6-pipecolinoxylidide hydrochloride vendor The FDA also said that the labeling of the diagnostic, which comes in different versions, included performance claims that did not match up with results seen in clinical studies—and that the data Innova submitted for review “was identical to data previously provided by other manufacturers” in separate requests for emergency COVID authorizations, raising additional questions. 2,6-pipecolinoxylidide hydrochloride factory The company’s product reserves are rich, with more than one hundred varieties in various categories from intermediates to APIs. At present, the grays and anti-hepatitis C series intermediates are steadily advancing, which will ensure future growth.

