We serve Chemical Name:D-(+)-Xylose CAS:6763-34-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
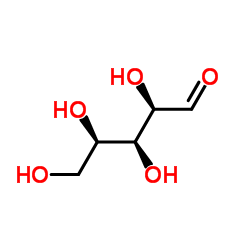
Chemical Name:D-(+)-Xylose
CAS.NO:6763-34-4
Synonyms:(D)-Xylose;Xylose;D-Xylose;Xylose, D-;aldehydo-D-xylose;(+)-Xylose;(2R,3S,4R)-2,3,4,5-Tetrahydroxypentanal
Molecular Formula:C5H10O5
Molecular Weight:150.130
HS Code:2940000000
Physical and Chemical Properties:
Melting point:148-153ºC
Boiling point:415.5±38.0 °C at 760 mmHg
Density:1.5±0.1 g/cm3
Index of Refraction:1.544
PSA:97.99000
Exact Mass:150.052826
LogP:-2.39
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (D)-Xylose chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(2R,3S,4R)-2,3,4,5-Tetrahydroxypentanal physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(+)-Xylose Use and application,(D)-Xylose technical grade,usp/ep/jp grade.
Related News: The FDA approval allows Biogen to sell its product over several years – with forecasts for potential annual sales reaching as high $10 billion to $50 billion – until the company completes a required follow-up study. D-(+)-Xylose manufacturer Fate Therapeutics is a clinical-stage biopharmaceutical company dedicated to the development of first-in-class cellular immunotherapies for cancer and immune disorders. D-(+)-Xylose supplier Fate Therapeutics is a clinical-stage biopharmaceutical company dedicated to the development of first-in-class cellular immunotherapies for cancer and immune disorders. D-(+)-Xylose vendor Rigosertib, in its intravenous formulation, is currently in Phase 3 clinical development for the treatment of higher-risk MDS. D-(+)-Xylose factory The FDA approval allows Biogen to sell its product over several years – with forecasts for potential annual sales reaching as high $10 billion to $50 billion – until the company completes a required follow-up study.

