We serve Chemical Name:cyclohexene-4,4,5,5-d4 CAS:68756-10-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
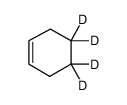
Chemical Name:cyclohexene-4,4,5,5-d4
CAS.NO:68756-10-5
Synonyms:cyclohexene-4,4,5,5-d4
Molecular Formula:C6H6D4
Molecular Weight:86.16820
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:
Exact Mass:86.10340
LogP:2.11660
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like cyclohexene-4,4,5,5-d4 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,cyclohexene-4,4,5,5-d4 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,cyclohexene-4,4,5,5-d4 Use and application,cyclohexene-4,4,5,5-d4 technical grade,usp/ep/jp grade.
Related News: There’s a few factors that play into this. cyclohexene-4,4,5,5-d4 manufacturer From the perspective of the overall life cycle of generic drugs from R & D to sales, with the expiration of patents, the business model for the production and marketing of generic pharmaceutical raw materials has changed, demand has increased, business potential has been realized quickly, and gross profit margins have shown a downward trend. cyclohexene-4,4,5,5-d4 supplier Johnson & Johnson’s Darzalex has comfortably been the only CD38 multiple myeloma drug on the market for years until Sanofi hit the scene last year with Sarclisa. Now, just as the competition’s slated to intensify, the U.S. company is building its case with new patient survival data. cyclohexene-4,4,5,5-d4 vendor There’s a few factors that play into this. cyclohexene-4,4,5,5-d4 factory Johnson & Johnson’s Darzalex has comfortably been the only CD38 multiple myeloma drug on the market for years until Sanofi hit the scene last year with Sarclisa. Now, just as the competition’s slated to intensify, the U.S. company is building its case with new patient survival data.

