We serve Chemical Name:Poly(Vinyl Silsesquioxane) CAS:69655-76-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
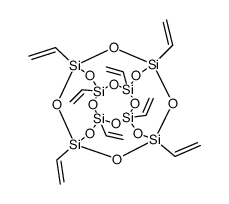
Chemical Name:Poly(Vinyl Silsesquioxane)
CAS.NO:69655-76-1
Synonyms:MFCD00217613;Octa(vinylsilasesquioxane);Poly(Vinyl Silsesquioxane)
Molecular Formula:C16H24O12Si8
Molecular Weight:633.03900
HS Code:
Physical and Chemical Properties:
Melting point:>350ºC
Boiling point:329.4ºC at 760mmHg
Density:1.22g/cm3
Index of Refraction:1.511
PSA:110.76000
Exact Mass:631.94200
LogP:1.56640
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like MFCD00217613 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Poly(Vinyl Silsesquioxane) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD00217613 Use and application,MFCD00217613 technical grade,usp/ep/jp grade.
Related News: An FDA inspection also turned up a long list of sanitary problems and bad manufacturing practices at the Emergent plant. Poly(Vinyl Silsesquioxane) manufacturer An FDA inspection also turned up a long list of sanitary problems and bad manufacturing practices at the Emergent plant. Poly(Vinyl Silsesquioxane) supplier An FDA inspection also turned up a long list of sanitary problems and bad manufacturing practices at the Emergent plant. Poly(Vinyl Silsesquioxane) vendor Delta moved the date up after the US State Department warned that people should not travel to China due to concerns about the spread of coronavirus, which was first discovered in Wuhan, Hubei province, in December. Poly(Vinyl Silsesquioxane) factory It is estimated that the size of China’s CMO market has reached about USD 5 billion. With the implementation and further implementation of the MAH system (the core is to separate drug marketing authorization from drug production authorization) in China, it is expected to promote the outbreak of the domestic drug CMO industry. It will grow at a rate of 20% to 30%, of which patented APIs occupy a larger proportion.

