We serve Chemical Name:gallium triflate CAS:74974-60-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
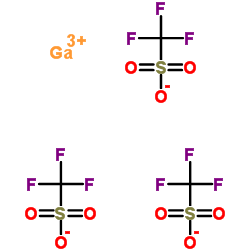
Chemical Name:gallium triflate
CAS.NO:74974-60-0
Synonyms:gallium triflate;gallium,trifluoromethanesulfonate;gallium(iii) triflate;Methanesulfonic acid, 1,1,1-trifluoro-, gallium salt (3:1);Gallium tris(trifluoromethanesulfonate)
Molecular Formula:C3H3F9GaO9S3
Molecular Weight:516.930
HS Code:2904909090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:162ºC at 760 mmHg
Density:1.7g/cm3
Index of Refraction:
PSA:196.74000
Exact Mass:515.781677
LogP:3.35330
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like gallium triflate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Gallium tris(trifluoromethanesulfonate) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,gallium(iii) triflate Use and application,Gallium tris(trifluoromethanesulfonate) technical grade,usp/ep/jp grade.
Related News: The main API products include antihypertensive, psychotropic and anti-AIDS special APIs. Antihypertensive APIs are mainly pulic and sartan drugs, and they are the world’s major suppliers of pulic and sartan APIs. gallium triflate manufacturer An effective treatment for Alzheimer��s, which is estimated in 60%-70% of around 50 million dementia cases worldwide, could become one of the best-selling drugs globally. gallium triflate supplier The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population. gallium triflate vendor The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population. gallium triflate factory The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population.

