We serve Chemical Name:hexadecanoic-16,16,16-d3 acid CAS:75736-53-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
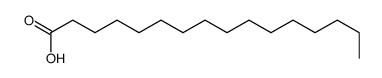
Chemical Name:hexadecanoic-16,16,16-d3 acid
CAS.NO:75736-53-7
Synonyms:Hexadecanoic acid-16,16,16-d3;Palmitic-d3 acid;16,16,16-trideuterio-hexadecanoic acid
Molecular Formula:C16H32O2
Molecular Weight:256.42400
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:37.30000
Exact Mass:256.24000
LogP:5.55230
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like Hexadecanoic acid-16,16,16-d3 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,16,16,16-trideuterio-hexadecanoic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,16,16,16-trideuterio-hexadecanoic acid Use and application,Hexadecanoic acid-16,16,16-d3 technical grade,usp/ep/jp grade.
Related News: This is very visible in herbal medicines in which the API is frequently a combination of several mixtures and/or substances which when used together cause pharmacological activity on the body. hexadecanoic-16,16,16-d3 acid manufacturer As part of the agreement, ICIG will enter into a 5-year supply contract to provide Genzyme with materials needed for the production of eliglustat tartrate, an investigational treatment for Gaucher disease Type 1 that is currently in Phase III clinical trials. hexadecanoic-16,16,16-d3 acid supplier We are pleased to work with Inceptua, given their strong record of administering such programs successfully.�� hexadecanoic-16,16,16-d3 acid vendor We are pleased to work with Inceptua, given their strong record of administering such programs successfully.�� hexadecanoic-16,16,16-d3 acid factory Under the terms of the agreement, ICIG will purchase substantially all of the pharmaceutical intermediates business, excluding the drug delivery technologies portion of the business.

