We serve Chemical Name:1,2-Nonanediol CAS:42789-13-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
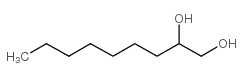
Chemical Name:1,2-Nonanediol
CAS.NO:42789-13-9
Synonyms:1,2-Dihydroxynonane;1,2-NONANEDIOL;nonane-1,2-diol
Molecular Formula:C9H20O2
Molecular Weight:160.25400
HS Code:2905399090
Physical and Chemical Properties:
Melting point:36ºC
Boiling point:155ºC / 17mmHg
Density:0.929g/cm3
Index of Refraction:1.454
PSA:40.46000
Exact Mass:160.14600
LogP:1.70010
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1,2-Dihydroxynonane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,nonane-1,2-diol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,2-NONANEDIOL Use and application,nonane-1,2-diol technical grade,usp/ep/jp grade.
Related News: An analysis of the top 20 global companies by R&D spend for 2018 shows that companies with expensive late-stage pipeline drugs are spending significant proportions of their annual revenue on R&D. 1,2-Nonanediol manufacturer In the process of fine chemical industry transfer, intermediates are a very important category, and they are also the main link for China to undertake transfers. 1,2-Nonanediol supplier An analysis of the top 20 global companies by R&D spend for 2018 shows that companies with expensive late-stage pipeline drugs are spending significant proportions of their annual revenue on R&D. 1,2-Nonanediol vendor From a global perspective, China’s API companies have also performed well. According to the report of the American Transparent Medicine website, in 2016 the top ten pharmaceutical companies in the global API market, Chinese pharmaceutical companies accounted for 6 seats. 1,2-Nonanediol factory The move follows a Class I recall, the FDA’s most serious, launched by Innova in late April amid “significant concerns” about the test’s accuracy—and alongside an official warning letter delivered to the company this week.

