We serve Chemical Name:1-(3-nitrophenyl)piperazine,dihydrochloride CAS:76835-12-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
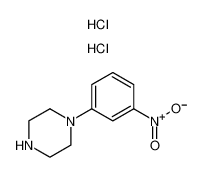
Chemical Name:1-(3-nitrophenyl)piperazine,dihydrochloride
CAS.NO:76835-12-6
Synonyms:1-(3-nitrophenyl)piperazine,dihydrochloride
Molecular Formula:C10H15Cl2N3O2
Molecular Weight:280.15100
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:61.09000
Exact Mass:279.05400
LogP:3.52540
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1-(3-nitrophenyl)piperazine,dihydrochloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1-(3-nitrophenyl)piperazine,dihydrochloride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1-(3-nitrophenyl)piperazine,dihydrochloride Use and application,1-(3-nitrophenyl)piperazine,dihydrochloride technical grade,usp/ep/jp grade.
Related News: IBI321 was discovered through a collaboration between Innovent and Eli Lilly and Company and has been developed in China by Innovent. The IND for IBI321 has been approved by the NMPA in China, and clinical trials in China are actively being conducted. Amino(3-thienyl)acetic acid manufacturers Today the international business represents 25 percent of BetterUp’s members, who access the platform through global enterprise partners including Allianz, Hilton, Snap Inc. and Mars. Boc-(R)-3-amino-4-(4-nitrophenyl)-butyric acid suppliers Inspired by the spirit of Start with Integrity, Succeed through Action,” Innovent’s mission is to develop, manufacture and commercialize high-quality biopharmaceutical products that are affordable to ordinary people.
Established in 2011, Innovent is committed to developing, manufacturing and commercializing high-quality innovative medicines for the treatment of cancer, autoimmune, metabolic and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since its inception, Innovent has developed a fully integrated multi-functional platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities.
Leveraging the platform, the company has built a robust pipeline of 25 valuable assets in the fields of cancer, metabolic, autoimmune disease and other major therapeutic areas, with 5 products – TYVYT® (sintilimab injection), BYVASDA® (bevacizumab biosimilar injection), SULINNO® (adalimumab biosimilar injection), HALPRYZA® (rituximab biosimilar injection) and Pemazyre® (pemigatinib oral inhibitor) – officially approved for marketing, 1 asset’s NDA under NMPA review, sintilimab’s Biologics License Application (BLA) acceptance in the U.S., 5 assets in Phase 3 or pivotal clinical trials, and an additional 14 molecules in clinical studies.
Innovent has built an international team with advanced talent in high-end biological drug development and commercialization, including many global experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Adimab, Incyte, MD Anderson Cancer Center, Hanmi and other international partners.
Innovent strives to work with many collaborators to help advance China’s biopharmaceutical industry, improve drug availability and enhance the quality of the patients’ lives. For more information, please visit 3-amino-4-(4-morpholino)benzotrifluoride vendor & factory.
Established in 2011, Innovent is committed to developing, manufacturing and commercializing high-quality innovative medicines for the treatment of cancer, autoimmune, metabolic and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since its inception, Innovent has developed a fully integrated multi-functional platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities.
Leveraging the platform, the company has built a robust pipeline of 25 valuable assets in the fields of cancer, metabolic, autoimmune disease and other major therapeutic areas, with 5 products – TYVYT® (sintilimab injection), BYVASDA® (bevacizumab biosimilar injection), SULINNO® (adalimumab biosimilar injection), HALPRYZA® (rituximab biosimilar injection) and Pemazyre® (pemigatinib oral inhibitor) – officially approved for marketing, 1 asset’s NDA under NMPA review, sintilimab’s Biologics License Application (BLA) acceptance in the U.S., 5 assets in Phase 3 or pivotal clinical trials, and an additional 14 molecules in clinical studies.
Innovent has built an international team with advanced talent in high-end biological drug development and commercialization, including many global experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Adimab, Incyte, MD Anderson Cancer Center, Hanmi and other international partners.
Innovent strives to work with many collaborators to help advance China’s biopharmaceutical industry, improve drug availability and enhance the quality of the patients’ lives. For more information, please visit 3-amino-4-(4-morpholino)benzotrifluoride vendor & factory.

