We serve Chemical Name:2-(Trifluoromethyl)-1H-imidazole-4,5-dicarboxylic acid CAS:78016-96-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
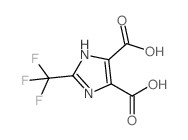
Chemical Name:2-(Trifluoromethyl)-1H-imidazole-4,5-dicarboxylic acid
CAS.NO:78016-96-3
Synonyms:2-trifluoromethylimidazole-4,5-dicarboxylic acid
Molecular Formula:C6H3F3N2O4
Molecular Weight:224.09400
HS Code:2933290090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:103.28000
Exact Mass:224.00400
LogP:0.82490
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-trifluoromethylimidazole-4,5-dicarboxylic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-trifluoromethylimidazole-4,5-dicarboxylic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-trifluoromethylimidazole-4,5-dicarboxylic acid Use and application,2-trifluoromethylimidazole-4,5-dicarboxylic acid technical grade,usp/ep/jp grade.
Related News: The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market. 2-(Trifluoromethyl)-1H-imidazole-4,5-dicarboxylic acid manufacturer With the advancement of supply-side structural reforms, the production of bulk APIs in China will become more concentrated, and the technology will be further improved. 2-(Trifluoromethyl)-1H-imidazole-4,5-dicarboxylic acid supplier The Basser Center launched its initiative Black & BRCA” in 2020 to bring tailored resources and support to the Black community for genetic counseling and testing.
“At a time when Black men and women are more likely to be diagnosed with cancer at later stages when it is less treatable, Black & BRCA seeks to empower people to understand their family health history and take action to prevent cancer from one generation to the next,” Domchek said.
Suffering through a case of COVID-19 unleashed a host of other health problems in hundreds of thousands of Americans participating in the largest study yet of the long-term effects of coronavirus infection.
Tracking the health insurance records of nearly 2 million people who caught the coronavirus last year, researchers found that one month or more after their infection, almost one-quarter of them sought medical treatment for new conditions, The New York Times reported.
The range of both those affected and the symptoms that struck them was wide. The health issues affected all ages, including children. The most common new health problems were pain; breathing difficulties; high cholesterol; malaise and fatigue; and high blood pressure. But symptoms did not stop there: Some suffered intestinal symptoms; migraines; skin problems; heart abnormalities; sleep disorders; and mental health conditions like anxiety and depression.
Post-COVID health problems did not spare those who had not been seriously ill: While nearly half of patients who were hospitalized for COVID-19 experienced subsequent medical issues, so did 27 percent of people who had mild or moderate symptoms and 19 percent of people who said they were asymptomatic.
“One thing that was surprising to us was the large percentage of asymptomatic patients that are in that category of long COVID,” Robin Gelburd, president of the nonprofit FAIR Health, told the Times.
Gelburd said that since asymptomatic people can have post-COVID symptoms, patients and doctors alike should consider the possibility that some health issues may actually be aftereffects of coronavirus infection.
In total, the report found that more than 454,000 people consulted health providers for symptoms 30 days or more after their infection. The analysis was evaluated by an independent academic reviewer but was not formally peer-reviewed, according to FAIR Health.
“The strength of this study is really its size and its ability to look across the range of disease severity in a diversity of age groups,” Dr. Helen Chu, an associate professor of medicine and infectious diseases at the University of Washington’s School of Medicine, told the Times.
The report “drives home the point that long COVID can affect nearly every organ system,” Dr. Ziyad Al-Aly, chief of the research and development service at the VA St. Louis Health Care System, told the Times.
“Some of these manifestations are chronic conditions that will last a lifetime and will forever scar some individuals and families,” added Al-Aly, who authored a large study published in April on lingering symptoms in COVID-19 patients in the Department of Veterans Affairs health system.
In the latest report, the most common issue for which patients sought medical care was pain — including nerve inflammation and aches and pains associated with nerves and muscles. It was reported by more than a fifth of those who reported post-COVID problems. Breathing difficulties, including shortness of breath, were experienced by 3.5 percent of post-COVID patients.
Nearly 3 percent of patients sought treatment for symptoms that were labeled with diagnostic codes for malaise and fatigue, a far-reaching category that could include issues like brain fog and exhaustion that worsens after physical or mental activity, the Times reported.
The database included only people with private health insurance or Medicare Advantage, not those uninsured or covered by Medicare Parts A, B and D, Medicaid or other government health programs. Chu told the Times that people without insurance or with incomes low enough to qualify for Medicaid are often “more likely to have worse outcomes. 2-(Trifluoromethyl)-1H-imidazole-4,5-dicarboxylic acid vendor With the advancement of supply-side structural reforms, the production of bulk APIs in China will become more concentrated, and the technology will be further improved. 2-(Trifluoromethyl)-1H-imidazole-4,5-dicarboxylic acid factory The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market.
“At a time when Black men and women are more likely to be diagnosed with cancer at later stages when it is less treatable, Black & BRCA seeks to empower people to understand their family health history and take action to prevent cancer from one generation to the next,” Domchek said.
Suffering through a case of COVID-19 unleashed a host of other health problems in hundreds of thousands of Americans participating in the largest study yet of the long-term effects of coronavirus infection.
Tracking the health insurance records of nearly 2 million people who caught the coronavirus last year, researchers found that one month or more after their infection, almost one-quarter of them sought medical treatment for new conditions, The New York Times reported.
The range of both those affected and the symptoms that struck them was wide. The health issues affected all ages, including children. The most common new health problems were pain; breathing difficulties; high cholesterol; malaise and fatigue; and high blood pressure. But symptoms did not stop there: Some suffered intestinal symptoms; migraines; skin problems; heart abnormalities; sleep disorders; and mental health conditions like anxiety and depression.
Post-COVID health problems did not spare those who had not been seriously ill: While nearly half of patients who were hospitalized for COVID-19 experienced subsequent medical issues, so did 27 percent of people who had mild or moderate symptoms and 19 percent of people who said they were asymptomatic.
“One thing that was surprising to us was the large percentage of asymptomatic patients that are in that category of long COVID,” Robin Gelburd, president of the nonprofit FAIR Health, told the Times.
Gelburd said that since asymptomatic people can have post-COVID symptoms, patients and doctors alike should consider the possibility that some health issues may actually be aftereffects of coronavirus infection.
In total, the report found that more than 454,000 people consulted health providers for symptoms 30 days or more after their infection. The analysis was evaluated by an independent academic reviewer but was not formally peer-reviewed, according to FAIR Health.
“The strength of this study is really its size and its ability to look across the range of disease severity in a diversity of age groups,” Dr. Helen Chu, an associate professor of medicine and infectious diseases at the University of Washington’s School of Medicine, told the Times.
The report “drives home the point that long COVID can affect nearly every organ system,” Dr. Ziyad Al-Aly, chief of the research and development service at the VA St. Louis Health Care System, told the Times.
“Some of these manifestations are chronic conditions that will last a lifetime and will forever scar some individuals and families,” added Al-Aly, who authored a large study published in April on lingering symptoms in COVID-19 patients in the Department of Veterans Affairs health system.
In the latest report, the most common issue for which patients sought medical care was pain — including nerve inflammation and aches and pains associated with nerves and muscles. It was reported by more than a fifth of those who reported post-COVID problems. Breathing difficulties, including shortness of breath, were experienced by 3.5 percent of post-COVID patients.
Nearly 3 percent of patients sought treatment for symptoms that were labeled with diagnostic codes for malaise and fatigue, a far-reaching category that could include issues like brain fog and exhaustion that worsens after physical or mental activity, the Times reported.
The database included only people with private health insurance or Medicare Advantage, not those uninsured or covered by Medicare Parts A, B and D, Medicaid or other government health programs. Chu told the Times that people without insurance or with incomes low enough to qualify for Medicaid are often “more likely to have worse outcomes. 2-(Trifluoromethyl)-1H-imidazole-4,5-dicarboxylic acid vendor With the advancement of supply-side structural reforms, the production of bulk APIs in China will become more concentrated, and the technology will be further improved. 2-(Trifluoromethyl)-1H-imidazole-4,5-dicarboxylic acid factory The FDA has granted so-called accelerated approval in more than 250 instances since 1992, mainly for rare diseases or small patient populations that have had no effective treatments available to them. In these cases, the agency requires that drugmakers conduct additional clinical trials to prove their therapy works, or face withdrawal from the market.

