We serve Chemical Name:Pigment Yellow 174 CAS:78952-72-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
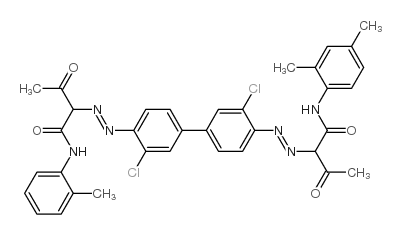
Chemical Name:Pigment Yellow 174
CAS.NO:78952-72-4
Synonyms:EINECS 279-017-2;Yellow GRY80;DIARYLIDE YELLOW GRY;DIARYLIDE YELLOW AAMX/AAOT
Molecular Formula:C35H32Cl2N6O4
Molecular Weight:671.57200
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:796.3ºC at 760 mmHg
Density:1.31
Index of Refraction:1.636
PSA:141.78000
Exact Mass:670.18600
LogP:9.09160
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like EINECS 279-017-2 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,DIARYLIDE YELLOW AAMX/AAOT physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,DIARYLIDE YELLOW AAMX/AAOT Use and application,DIARYLIDE YELLOW GRY technical grade,usp/ep/jp grade.
Related News: We manufacture APIs and drug manufacturers make medicines from APIs. Pigment Yellow 174 manufacturer Safety concerns about the J&J vaccine paired with flagging U.S. demand for vaccinations in general have slowed rollout of the one-shot vaccine to a crawl. Close to half of the 21 million doses produced for the United States sit unused. Pigment Yellow 174 supplier It has reserves in the direction of APIs and intermediates for lowering blood lipids, lowering blood sugar and anticoagulation. Pigment Yellow 174 vendor Citizens and residents will be allowed entry to New Zealand, but will be required to quarantine themselves for 14 days, Prime Minister Jacinda Ardern said. Pigment Yellow 174 factory Darzalex has its own VRd combo trials, dubbed PERSEUS and CEPHEUS, which are testing a newly approved under-the-skin version of the J&J drug called Darzalex Faspro. The CEPHEUS trial appears to have just completed its primary analysis, according to a listing on ClinicalTrials.gov.

