We serve Chemical Name:4-(piperidin-2-ylmethyl)morpholine CAS:81310-58-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
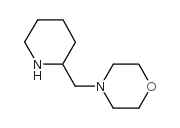
Chemical Name:4-(piperidin-2-ylmethyl)morpholine
CAS.NO:81310-58-9
Synonyms:4-Piperidin-2-ylmethylmorpholine
Molecular Formula:C10H20N2O
Molecular Weight:184.27900
HS Code:2934999090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:275.1ºC at 760 mmHg
Density:0.997g/cm3
Index of Refraction:1.482
PSA:24.50000
Exact Mass:184.15800
LogP:0.72740
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 4-Piperidin-2-ylmethylmorpholine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-Piperidin-2-ylmethylmorpholine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-Piperidin-2-ylmethylmorpholine Use and application,4-Piperidin-2-ylmethylmorpholine technical grade,usp/ep/jp grade.
Related News: From the three-month point onward, 84.5% of uMRD patients maintained that deep remission in the blood to the 12-month assessment. These data show the new combo can deliver “very durable remissions” for the vast majority of patients, Tendler said. 4-(piperidin-2-ylmethyl)morpholine manufacturer First up is Lilly, which reached all-time highs this week as attention turned to its once highly hyped donanemab, another anti-amyloid that saw a mixed bag of data back in March, with a slight win on one disease scale undermined by a failure on a more widely used measure of Alzheimer’s. 4-(piperidin-2-ylmethyl)morpholine supplier Last year, California became the first state in the country to ban toxic chemicals in cosmetics — including a number of PFAS. Maryland did the same this month, but the laws don’t take effect until 2025, the Post reported. 4-(piperidin-2-ylmethyl)morpholine vendor Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely. 4-(piperidin-2-ylmethyl)morpholine factory This is particularly important in reducing overall disease burden. Moreover, this strategic decision will not only strengthen our diabetes portfolio but also help consolidate our position as the fastest-growing player in the anti-diabetes segment, Mankind Pharma Director of Marketing Sanjay Koul said in a statement.

