We serve Chemical Name:Cyclopentanol, 2-(1-piperazinyl)-, (1R,2R) CAS:815632-26-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
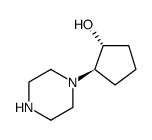
Chemical Name:Cyclopentanol, 2-(1-piperazinyl)-, (1R,2R)
CAS.NO:815632-26-9
Synonyms:(1R,2R)-2-PIPERAZIN-1-YLCYCLOPENTANOL;(1R,2R)-2-(1-piperazinyl)cyclopentanol
Molecular Formula:C9H18N2O
Molecular Weight:170.25200
HS Code:2933599090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:35.50000
Exact Mass:170.14200
LogP:0.07170
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (1R,2R)-2-PIPERAZIN-1-YLCYCLOPENTANOL chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(1R,2R)-2-(1-piperazinyl)cyclopentanol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(1R,2R)-2-PIPERAZIN-1-YLCYCLOPENTANOL Use and application,(1R,2R)-2-PIPERAZIN-1-YLCYCLOPENTANOL technical grade,usp/ep/jp grade.
Related News: In recent years, there has been a relatively concentrated concentration of intermediate companies listed on the capital market. Most of the fund-raising projects are aimed at breaking through the bottleneck of production capacity to meet growing demand, and the entire industry has shown a vigorous development momentum. Cyclopentanol, 2-(1-piperazinyl)-, (1R,2R) manufacturer In an August overhaul to its drug administration law, Beijing said conditional approval could be granted to some still-under-research medicines of ��predictable�� clinical value for life-threatening diseases for which effective treatment is not immediately available. Cyclopentanol, 2-(1-piperazinyl)-, (1R,2R) supplier Taking Minuo Huawei as an example, in 2016, the capacity utilization rate and production-sales ratio of its multiple drug substance varieties reached more than 90%. Therefore, domestic API companies have expanded their production capacity in recent years to meet the growing demand for APIs. Cyclopentanol, 2-(1-piperazinyl)-, (1R,2R) vendor This suspension applies to all dogs, including puppies, emotional support dogs, and dogs that traveled out of the United States and are returning from a high-risk country,” the CDC said in a statement.
The agency said dog rescue missions, imports from dog breeders and people bringing in pets will be affected by the decision, CNN reported.
“If these dogs coming from high-risk countries haven’t been properly vaccinated, there is a risk they could bring it into the country,” Dr. Emily Pieracci, a veterinary medical officer in CDC’s Division of Global Migration and Quarantine, told CNN.
“I think it is important to stress that this is a temporary suspension. We recognize that this is not the long-term solution,” she said, with the initial suspension likely to be 12 months.
Eton Pharmaceuticals, Inc (Nasdaq: ETON) today announced that the U.S. Food and Drug Administration (FDA) has approved Rezipres (ephedrine hydrochloride injection) for the treatment of clinically important hypotension occurring in the setting of anesthesia.
“We are excited to see the approval of Rezipres, which is now our second FDA-approved ready-to-use hospital injectable product. We believe ready-to-use injectable products provide a compelling benefit to hospitals and reduce the need for hospitals to rely on unapproved compounded products,” said Sean Brynjelsen, CEO of Eton Pharmaceuticals. “This innovative sulfite-free formulation has been successfully sold in Europe for years, and we are excited to make it available to U.S. patients shortly.”
Eton Pharmaceuticals, Inc. is an innovative pharmaceutical company focused on developing and commercializing treatments for rare diseases. The company currently owns or receives royalties from four FDA-approved products, including ALKINDI® SPRINKLE, Biorphen®, Rezipres®, and Alaway® Preservative Free, and has five additional products that have been submitted to the FDA.
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements associated with the expected ability of Eton to undertake certain activities and accomplish certain goals and objectives.
These statements include but are not limited to statements regarding Eton’s business strategy, Eton’s plans to develop and commercialize its product candidates, the safety and efficacy of Eton’s product candidates, Eton’s plans and expected timing with respect to regulatory filings and approvals, and the size and growth potential of the markets for Eton’s product candidates. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements.
These forward-looking statements are based upon Eton’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs.
These and other risks concerning Eton’s development programs and financial position are described in additional detail in Eton’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made.
Eton undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were identified in individuals before the first cases of infection were recognized, according to a study published online June 15 in Clinical Infectious Diseases.
Keri N. Althoff, Ph.D., M.P.H., from the Johns Hopkins Bloomberg School of Public Health in Baltimore, and colleagues aimed to identify individuals with SARS-CoV-2 antibodies in the early weeks of the U.S. pandemic among All of Us participants in all 50 U.S. states who provided blood specimens from Jan. 2 to March 18, 2020.
Participants were considered seropositive if they tested positive for SARS-CoV-2 immunoglobulin G antibodies with the Abbott or EUROIMMUN assays in a sequential testing algorithm.
The researchers found that the estimated sensitivity and specificity of Abbott were100 and 99.5 percent, respectively, and the estimated sensitivity and specificity of EUROIMMUN were 90.7 and 99.7 percent, respectively.
For the sequential testing algorithm used in the study, the net sensitivity and specificity were 90.7 and 100.0 percent, respectively. Overall, nine of the 24,079 study participants with blood specimens from Jan. 2 to March 18, 2020, were seropositive; seven of these were seropositive prior to the first confirmed case in the states of Illinois, Massachusetts, Wisconsin, Pennsylvania, and Mississippi.
“This study contributes to the evidence of low-level circulation of SARS-CoV-2 in many states at the start of the U.S. epidemic,” the authors write. “Future pandemic management should carefully consider the impact of epidemiologic links in testing recommendations and reduce testing restrictions as early as possible. Cyclopentanol, 2-(1-piperazinyl)-, (1R,2R) factory This suspension applies to all dogs, including puppies, emotional support dogs, and dogs that traveled out of the United States and are returning from a high-risk country,” the CDC said in a statement.
The agency said dog rescue missions, imports from dog breeders and people bringing in pets will be affected by the decision, CNN reported.
“If these dogs coming from high-risk countries haven’t been properly vaccinated, there is a risk they could bring it into the country,” Dr. Emily Pieracci, a veterinary medical officer in CDC’s Division of Global Migration and Quarantine, told CNN.
“I think it is important to stress that this is a temporary suspension. We recognize that this is not the long-term solution,” she said, with the initial suspension likely to be 12 months.
Eton Pharmaceuticals, Inc (Nasdaq: ETON) today announced that the U.S. Food and Drug Administration (FDA) has approved Rezipres (ephedrine hydrochloride injection) for the treatment of clinically important hypotension occurring in the setting of anesthesia.
“We are excited to see the approval of Rezipres, which is now our second FDA-approved ready-to-use hospital injectable product. We believe ready-to-use injectable products provide a compelling benefit to hospitals and reduce the need for hospitals to rely on unapproved compounded products,” said Sean Brynjelsen, CEO of Eton Pharmaceuticals. “This innovative sulfite-free formulation has been successfully sold in Europe for years, and we are excited to make it available to U.S. patients shortly.”
Eton Pharmaceuticals, Inc. is an innovative pharmaceutical company focused on developing and commercializing treatments for rare diseases. The company currently owns or receives royalties from four FDA-approved products, including ALKINDI® SPRINKLE, Biorphen®, Rezipres®, and Alaway® Preservative Free, and has five additional products that have been submitted to the FDA.
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements associated with the expected ability of Eton to undertake certain activities and accomplish certain goals and objectives.
These statements include but are not limited to statements regarding Eton’s business strategy, Eton’s plans to develop and commercialize its product candidates, the safety and efficacy of Eton’s product candidates, Eton’s plans and expected timing with respect to regulatory filings and approvals, and the size and growth potential of the markets for Eton’s product candidates. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements.
These forward-looking statements are based upon Eton’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs.
These and other risks concerning Eton’s development programs and financial position are described in additional detail in Eton’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made.
Eton undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were identified in individuals before the first cases of infection were recognized, according to a study published online June 15 in Clinical Infectious Diseases.
Keri N. Althoff, Ph.D., M.P.H., from the Johns Hopkins Bloomberg School of Public Health in Baltimore, and colleagues aimed to identify individuals with SARS-CoV-2 antibodies in the early weeks of the U.S. pandemic among All of Us participants in all 50 U.S. states who provided blood specimens from Jan. 2 to March 18, 2020.
Participants were considered seropositive if they tested positive for SARS-CoV-2 immunoglobulin G antibodies with the Abbott or EUROIMMUN assays in a sequential testing algorithm.
The researchers found that the estimated sensitivity and specificity of Abbott were100 and 99.5 percent, respectively, and the estimated sensitivity and specificity of EUROIMMUN were 90.7 and 99.7 percent, respectively.
For the sequential testing algorithm used in the study, the net sensitivity and specificity were 90.7 and 100.0 percent, respectively. Overall, nine of the 24,079 study participants with blood specimens from Jan. 2 to March 18, 2020, were seropositive; seven of these were seropositive prior to the first confirmed case in the states of Illinois, Massachusetts, Wisconsin, Pennsylvania, and Mississippi.
“This study contributes to the evidence of low-level circulation of SARS-CoV-2 in many states at the start of the U.S. epidemic,” the authors write. “Future pandemic management should carefully consider the impact of epidemiologic links in testing recommendations and reduce testing restrictions as early as possible.
The agency said dog rescue missions, imports from dog breeders and people bringing in pets will be affected by the decision, CNN reported.
“If these dogs coming from high-risk countries haven’t been properly vaccinated, there is a risk they could bring it into the country,” Dr. Emily Pieracci, a veterinary medical officer in CDC’s Division of Global Migration and Quarantine, told CNN.
“I think it is important to stress that this is a temporary suspension. We recognize that this is not the long-term solution,” she said, with the initial suspension likely to be 12 months.
Eton Pharmaceuticals, Inc (Nasdaq: ETON) today announced that the U.S. Food and Drug Administration (FDA) has approved Rezipres (ephedrine hydrochloride injection) for the treatment of clinically important hypotension occurring in the setting of anesthesia.
“We are excited to see the approval of Rezipres, which is now our second FDA-approved ready-to-use hospital injectable product. We believe ready-to-use injectable products provide a compelling benefit to hospitals and reduce the need for hospitals to rely on unapproved compounded products,” said Sean Brynjelsen, CEO of Eton Pharmaceuticals. “This innovative sulfite-free formulation has been successfully sold in Europe for years, and we are excited to make it available to U.S. patients shortly.”
Eton Pharmaceuticals, Inc. is an innovative pharmaceutical company focused on developing and commercializing treatments for rare diseases. The company currently owns or receives royalties from four FDA-approved products, including ALKINDI® SPRINKLE, Biorphen®, Rezipres®, and Alaway® Preservative Free, and has five additional products that have been submitted to the FDA.
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements associated with the expected ability of Eton to undertake certain activities and accomplish certain goals and objectives.
These statements include but are not limited to statements regarding Eton’s business strategy, Eton’s plans to develop and commercialize its product candidates, the safety and efficacy of Eton’s product candidates, Eton’s plans and expected timing with respect to regulatory filings and approvals, and the size and growth potential of the markets for Eton’s product candidates. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements.
These forward-looking statements are based upon Eton’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs.
These and other risks concerning Eton’s development programs and financial position are described in additional detail in Eton’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made.
Eton undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were identified in individuals before the first cases of infection were recognized, according to a study published online June 15 in Clinical Infectious Diseases.
Keri N. Althoff, Ph.D., M.P.H., from the Johns Hopkins Bloomberg School of Public Health in Baltimore, and colleagues aimed to identify individuals with SARS-CoV-2 antibodies in the early weeks of the U.S. pandemic among All of Us participants in all 50 U.S. states who provided blood specimens from Jan. 2 to March 18, 2020.
Participants were considered seropositive if they tested positive for SARS-CoV-2 immunoglobulin G antibodies with the Abbott or EUROIMMUN assays in a sequential testing algorithm.
The researchers found that the estimated sensitivity and specificity of Abbott were100 and 99.5 percent, respectively, and the estimated sensitivity and specificity of EUROIMMUN were 90.7 and 99.7 percent, respectively.
For the sequential testing algorithm used in the study, the net sensitivity and specificity were 90.7 and 100.0 percent, respectively. Overall, nine of the 24,079 study participants with blood specimens from Jan. 2 to March 18, 2020, were seropositive; seven of these were seropositive prior to the first confirmed case in the states of Illinois, Massachusetts, Wisconsin, Pennsylvania, and Mississippi.
“This study contributes to the evidence of low-level circulation of SARS-CoV-2 in many states at the start of the U.S. epidemic,” the authors write. “Future pandemic management should carefully consider the impact of epidemiologic links in testing recommendations and reduce testing restrictions as early as possible. Cyclopentanol, 2-(1-piperazinyl)-, (1R,2R) factory This suspension applies to all dogs, including puppies, emotional support dogs, and dogs that traveled out of the United States and are returning from a high-risk country,” the CDC said in a statement.
The agency said dog rescue missions, imports from dog breeders and people bringing in pets will be affected by the decision, CNN reported.
“If these dogs coming from high-risk countries haven’t been properly vaccinated, there is a risk they could bring it into the country,” Dr. Emily Pieracci, a veterinary medical officer in CDC’s Division of Global Migration and Quarantine, told CNN.
“I think it is important to stress that this is a temporary suspension. We recognize that this is not the long-term solution,” she said, with the initial suspension likely to be 12 months.
Eton Pharmaceuticals, Inc (Nasdaq: ETON) today announced that the U.S. Food and Drug Administration (FDA) has approved Rezipres (ephedrine hydrochloride injection) for the treatment of clinically important hypotension occurring in the setting of anesthesia.
“We are excited to see the approval of Rezipres, which is now our second FDA-approved ready-to-use hospital injectable product. We believe ready-to-use injectable products provide a compelling benefit to hospitals and reduce the need for hospitals to rely on unapproved compounded products,” said Sean Brynjelsen, CEO of Eton Pharmaceuticals. “This innovative sulfite-free formulation has been successfully sold in Europe for years, and we are excited to make it available to U.S. patients shortly.”
Eton Pharmaceuticals, Inc. is an innovative pharmaceutical company focused on developing and commercializing treatments for rare diseases. The company currently owns or receives royalties from four FDA-approved products, including ALKINDI® SPRINKLE, Biorphen®, Rezipres®, and Alaway® Preservative Free, and has five additional products that have been submitted to the FDA.
Statements contained in this press release regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements associated with the expected ability of Eton to undertake certain activities and accomplish certain goals and objectives.
These statements include but are not limited to statements regarding Eton’s business strategy, Eton’s plans to develop and commercialize its product candidates, the safety and efficacy of Eton’s product candidates, Eton’s plans and expected timing with respect to regulatory filings and approvals, and the size and growth potential of the markets for Eton’s product candidates. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “believes,” “anticipates,” “plans,” “expects,” “intends,” “will,” “goal,” “potential” and similar expressions are intended to identify forward-looking statements.
These forward-looking statements are based upon Eton’s current expectations and involve assumptions that may never materialize or may prove to be incorrect.
Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, risks associated with the process of discovering, developing and commercializing drugs that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs.
These and other risks concerning Eton’s development programs and financial position are described in additional detail in Eton’s filings with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made.
Eton undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies were identified in individuals before the first cases of infection were recognized, according to a study published online June 15 in Clinical Infectious Diseases.
Keri N. Althoff, Ph.D., M.P.H., from the Johns Hopkins Bloomberg School of Public Health in Baltimore, and colleagues aimed to identify individuals with SARS-CoV-2 antibodies in the early weeks of the U.S. pandemic among All of Us participants in all 50 U.S. states who provided blood specimens from Jan. 2 to March 18, 2020.
Participants were considered seropositive if they tested positive for SARS-CoV-2 immunoglobulin G antibodies with the Abbott or EUROIMMUN assays in a sequential testing algorithm.
The researchers found that the estimated sensitivity and specificity of Abbott were100 and 99.5 percent, respectively, and the estimated sensitivity and specificity of EUROIMMUN were 90.7 and 99.7 percent, respectively.
For the sequential testing algorithm used in the study, the net sensitivity and specificity were 90.7 and 100.0 percent, respectively. Overall, nine of the 24,079 study participants with blood specimens from Jan. 2 to March 18, 2020, were seropositive; seven of these were seropositive prior to the first confirmed case in the states of Illinois, Massachusetts, Wisconsin, Pennsylvania, and Mississippi.
“This study contributes to the evidence of low-level circulation of SARS-CoV-2 in many states at the start of the U.S. epidemic,” the authors write. “Future pandemic management should carefully consider the impact of epidemiologic links in testing recommendations and reduce testing restrictions as early as possible.

