We serve Chemical Name:3-Butoxy-N,N-dimethylpropanamide CAS:845544-42-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
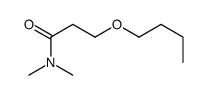
Chemical Name:3-Butoxy-N,N-dimethylpropanamide
CAS.NO:845544-42-5
Synonyms:3-Butoxy-N,N-dimethylpropanamide
Molecular Formula:C9H19NO2
Molecular Weight:173.25300
HS Code:2924199090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:29.54000
Exact Mass:173.14200
LogP:1.28140
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 3-Butoxy-N,N-dimethylpropanamide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-Butoxy-N,N-dimethylpropanamide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Butoxy-N,N-dimethylpropanamide Use and application,3-Butoxy-N,N-dimethylpropanamide technical grade,usp/ep/jp grade.
Related News: Vietnam backtracked on Saturday and narrowed its restrictions to most flights from mainland China. 3-Butoxy-N,N-dimethylpropanamide manufacturer The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population. 3-Butoxy-N,N-dimethylpropanamide supplier GlaxoSmithKline��s HIV drugs division ViiV Healthcare said on Saturday that the U.S. Food and Drug Administration declined to approve its long acting HIV injection. 3-Butoxy-N,N-dimethylpropanamide vendor The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population. 3-Butoxy-N,N-dimethylpropanamide factory GlaxoSmithKline��s HIV drugs division ViiV Healthcare said on Saturday that the U.S. Food and Drug Administration declined to approve its long acting HIV injection.

