We serve Chemical Name:2-Fluoro-6-iodophenylboronic acid CAS:870777-22-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
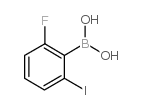
Chemical Name:2-Fluoro-6-iodophenylboronic acid
CAS.NO:870777-22-3
Synonyms:(2-fluoro-6-iodophenyl)boronic acid
Molecular Formula:C6H5BFIO2
Molecular Weight:265.81700
HS Code:2931900090
Physical and Chemical Properties:
Melting point:135-139ºC(lit.)
Boiling point:338.3ºC at 760 mmHg
Density:2.01g/cm3
Index of Refraction:1.621
PSA:40.46000
Exact Mass:265.94100
LogP:0.11010
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like (2-fluoro-6-iodophenyl)boronic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(2-fluoro-6-iodophenyl)boronic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(2-fluoro-6-iodophenyl)boronic acid Use and application,(2-fluoro-6-iodophenyl)boronic acid technical grade,usp/ep/jp grade.
Related News: Recipharm joined the COVID-19 fight in December, signing on to produce the Moderna vaccine at its plant in Monts, France. The company hasn’t revealed if the new facilities will be engaged in COVID-19 vaccine production, though the plant in Morocco will “mirror” a new fill-finish line it added in France. 2-[(6-butoxypyridin-3-yl)amino]-4-chlorobenzoic acid manufacturers Walmsley’s background in consumer health—and lack of prior experience in pharma—has reportedly been targeted by Elliott in the investment firm’s secret push for a new boss at GSK. 6-hydroxy-4-methyl-7-(1-phenyl-allyl)-chromen-2-one suppliers The results provided a formulation amenable to two dosage strengths by applying a proportional dosing weight method.” The product is currently in late-stage development with the New Drug Application filing expected in late 2021. 5-(3-methoxystyryl)-1,3,4-thiadiazol-2-amine vendor & factory The results provided a formulation amenable to two dosage strengths by applying a proportional dosing weight method.” The product is currently in late-stage development with the New Drug Application filing expected in late 2021.,Recipharm joined the COVID-19 fight in December, signing on to produce the Moderna vaccine at its plant in Monts, France. The company hasn’t revealed if the new facilities will be engaged in COVID-19 vaccine production, though the plant in Morocco will “mirror” a new fill-finish line it added in France.

