We serve Chemical Name:3-Bromo-2-fluorobenzamide CAS:871353-25-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
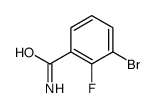
Chemical Name:3-Bromo-2-fluorobenzamide
CAS.NO:871353-25-2
Synonyms:3-Bromo-2-fluorobenzamide
Molecular Formula:C7H5BrFNO
Molecular Weight:218.02300
HS Code:2924299090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:44.08000
Exact Mass:216.95400
LogP:2.57130
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 3-Bromo-2-fluorobenzamide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-Bromo-2-fluorobenzamide physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Bromo-2-fluorobenzamide Use and application,3-Bromo-2-fluorobenzamide technical grade,usp/ep/jp grade.
Related News: The company reported that it was cooperating with the Justice Department probe and that an outside law firm was also “investigating these allegations thoroughly.” 3-Bromo-2-fluorobenzamide manufacturer The workers are demanding that Hong Kong close all border checkpoints to visitors from mainland China, saying they represent a threat to health care workers in the city. 3-Bromo-2-fluorobenzamide supplier The company reported that it was cooperating with the Justice Department probe and that an outside law firm was also “investigating these allegations thoroughly.” 3-Bromo-2-fluorobenzamide vendor The Company��s first-of-kind approach involves engineering human iPSCs in a one-time genetic modification event and selecting a single engineered iPSC for maintenance as a clonal master iPSC line. 3-Bromo-2-fluorobenzamide factory Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.

