We serve Chemical Name:ethyl 4-pyridin-2-ylbutanimidate CAS:887579-19-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
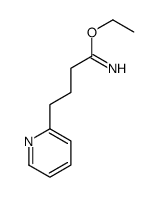
Chemical Name:ethyl 4-pyridin-2-ylbutanimidate
CAS.NO:887579-19-3
Synonyms:2-Pyridinebutanimidicacid,ethyl ester;4-PYRIDIN-2-YL-BUTYRIMIDIC ACID ETHYL ESTER
Molecular Formula:C11H16N2O
Molecular Weight:192.25800
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:45.97000
Exact Mass:192.12600
LogP:2.51780
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-Pyridinebutanimidicacid,ethyl ester chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-PYRIDIN-2-YL-BUTYRIMIDIC ACID ETHYL ESTER physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Pyridinebutanimidicacid,ethyl ester Use and application,2-Pyridinebutanimidicacid,ethyl ester technical grade,usp/ep/jp grade.
Related News: An FDA inspection also turned up a long list of sanitary problems and bad manufacturing practices at the Emergent plant. ethyl 4-pyridin-2-ylbutanimidate manufacturer The European Medicines Agency did not say how many shots were affected, but Reuters has reported it involves millions of doses, making it harder for J&J to meet a target of delivering 55 million to Europe by end of June. ethyl 4-pyridin-2-ylbutanimidate supplier Last year, California became the first state in the country to ban toxic chemicals in cosmetics — including a number of PFAS. Maryland did the same this month, but the laws don’t take effect until 2025, the Post reported. ethyl 4-pyridin-2-ylbutanimidate vendor When they do design their phase 3, they’re going to need to be better about that part of the study design, and really focus on enrolling individuals that have the right biomarkers to be able to look at not only the safety and tolerability but also the efficacy on cognition and function,” Edelmayer said.
The vaccine approach is promising because using the body’s immune system to fight Alzheimer’s would sidestep one of the problems in developing a drug to treat the disease — namely that it’s difficult to design medications that can easily enter the brain and attack a specific target, Edelmayer explained.
Through booster doses given every three months, the vaccine is “training your body over time how to react to the pathological tau,” Edelmayer said. “You will likely need additional shots of this particular therapeutic to keep it as productive as possible in targeting that tau. ethyl 4-pyridin-2-ylbutanimidate factory When they do design their phase 3, they’re going to need to be better about that part of the study design, and really focus on enrolling individuals that have the right biomarkers to be able to look at not only the safety and tolerability but also the efficacy on cognition and function,” Edelmayer said.
The vaccine approach is promising because using the body’s immune system to fight Alzheimer’s would sidestep one of the problems in developing a drug to treat the disease — namely that it’s difficult to design medications that can easily enter the brain and attack a specific target, Edelmayer explained.
Through booster doses given every three months, the vaccine is “training your body over time how to react to the pathological tau,” Edelmayer said. “You will likely need additional shots of this particular therapeutic to keep it as productive as possible in targeting that tau.
The vaccine approach is promising because using the body’s immune system to fight Alzheimer’s would sidestep one of the problems in developing a drug to treat the disease — namely that it’s difficult to design medications that can easily enter the brain and attack a specific target, Edelmayer explained.
Through booster doses given every three months, the vaccine is “training your body over time how to react to the pathological tau,” Edelmayer said. “You will likely need additional shots of this particular therapeutic to keep it as productive as possible in targeting that tau. ethyl 4-pyridin-2-ylbutanimidate factory When they do design their phase 3, they’re going to need to be better about that part of the study design, and really focus on enrolling individuals that have the right biomarkers to be able to look at not only the safety and tolerability but also the efficacy on cognition and function,” Edelmayer said.
The vaccine approach is promising because using the body’s immune system to fight Alzheimer’s would sidestep one of the problems in developing a drug to treat the disease — namely that it’s difficult to design medications that can easily enter the brain and attack a specific target, Edelmayer explained.
Through booster doses given every three months, the vaccine is “training your body over time how to react to the pathological tau,” Edelmayer said. “You will likely need additional shots of this particular therapeutic to keep it as productive as possible in targeting that tau.

