We serve Chemical Name:Maltodextrin CAS:9050-36-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
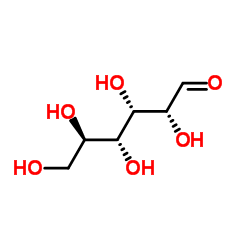
Chemical Name:Maltodextrin
CAS.NO:9050-36-6
Synonyms:EINECS 232-940-4;MFCD00146679;Maltodextrin
Molecular Formula:C6H12O6
Molecular Weight:180.156
HS Code:3505100000
Physical and Chemical Properties:
Melting point:240ºC (dec.)
Boiling point:527.1±50.0 °C at 760 mmHg
Density:1.6±0.1 g/cm3
Index of Refraction:1.573
PSA:118.22000
Exact Mass:180.063385
LogP:-3.17
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like EINECS 232-940-4 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Maltodextrin physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Maltodextrin Use and application,EINECS 232-940-4 technical grade,usp/ep/jp grade.
Related News: The FDA approval allows Biogen to sell its product over several years – with forecasts for potential annual sales reaching as high $10 billion to $50 billion – until the company completes a required follow-up study. Maltodextrin manufacturer Cases recorded in Thailand, Taiwan, Germany, Vietnam, Japan, France and the United States involved patients who had not been to China. Maltodextrin supplier The vaccine also proved safe during the two-year trial, in which eleven doses were administered to randomly chosen patients with mild dementia. People who received the vaccine, known as AADvac1, experienced about the same numbers of side effects and adverse events as those who were given a placebo. Maltodextrin vendor The FDA approval allows Biogen to sell its product over several years – with forecasts for potential annual sales reaching as high $10 billion to $50 billion – until the company completes a required follow-up study. Maltodextrin factory Patents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.

