We serve Chemical Name:quinmerac CAS:90717-03-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
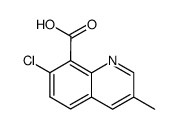
Chemical Name:quinmerac
CAS.NO:90717-03-6
Synonyms:8-QUINOLINECARBOXYLICACID, 7-CHLORO-3-METHYL-;Quinmerac;BAS 518;3-methyl-7-chloroquinoline-8-carboxylic acid;8-Quinolinecarboxylic acid,7-chloro-3-methyl;BAS 518H;UNII-0OFY83UPMH;PROCHLORPERAZINE DIMESYLATE;7-chloro-3-methylquinoline-8-carboxylic acid;7-chloro-3-methyl-8-quinolinecarboxylic acid;7-chloro-3-methyl-quinoline-8-carboxylic acid
Molecular Formula:C11H8ClNO2
Molecular Weight:221.64000
HS Code:2933499013
Physical and Chemical Properties:
Melting point:244ºC
Boiling point:416ºC at 760 mmHg
Density:1.406g/cm3
Index of Refraction:1.669
PSA:50.19000
Exact Mass:221.02400
LogP:2.89480
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like 8-QUINOLINECARBOXYLICACID, 7-CHLORO-3-METHYL- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,7-chloro-3-methyl-quinoline-8-carboxylic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,BAS 518H Use and application,7-chloro-3-methyl-quinoline-8-carboxylic acid technical grade,usp/ep/jp grade.
Related News: Prime Minister Scott Morrison said Saturday that all travelers from mainland China allowed into Australia had to be quarantined for 14 days. quinmerac manufacturer If the results from that trial are positive, the company might apply to the U.S. Food and Drug Administration for the same accelerated approval pathway recently used to bring the controversial Alzheimer’s drug aducanumab to market, Novak said. quinmerac supplier The Branchburg factory first came under FDA scrutiny in late 2019, when agency inspectors began to document numerous quality control problems. By March, 2020, the FDA had deemed the manufacturing issues as “Official Action Indicated,” its most serious category of violation. quinmerac vendor The Branchburg factory first came under FDA scrutiny in late 2019, when agency inspectors began to document numerous quality control problems. By March, 2020, the FDA had deemed the manufacturing issues as “Official Action Indicated,” its most serious category of violation. quinmerac factory The Branchburg factory first came under FDA scrutiny in late 2019, when agency inspectors began to document numerous quality control problems. By March, 2020, the FDA had deemed the manufacturing issues as “Official Action Indicated,” its most serious category of violation.

