We serve Chemical Name:chlorogold,tris(2,4-ditert-butylphenyl) phosphite CAS:915299-24-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
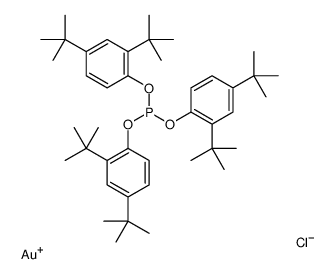
Chemical Name:chlorogold,tris(2,4-ditert-butylphenyl) phosphite
CAS.NO:915299-24-0
Synonyms:chlorogold,tris(2,4-ditert-butylphenyl) phosphite
Molecular Formula:C42H63AuClO3P
Molecular Weight:879.34100
HS Code:
Physical and Chemical Properties:
Melting point:200-202ºC
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:41.28000
Exact Mass:878.38700
LogP:10.23680
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like chlorogold,tris(2,4-ditert-butylphenyl) phosphite chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,chlorogold,tris(2,4-ditert-butylphenyl) phosphite physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,chlorogold,tris(2,4-ditert-butylphenyl) phosphite Use and application,chlorogold,tris(2,4-ditert-butylphenyl) phosphite technical grade,usp/ep/jp grade.
Related News: At present, the additional 300 tons of sunniamine, 300 tons of fluoxamic acid, and 200 tons of cyprodinil have been successfully put into production, and the profitability is good. chlorogold,tris(2,4-ditert-butylphenyl) phosphite manufacturer In the TOP50 list of API export companies in 2017, the geographical clustering of advantageous companies is still obvious. For example, Huahai and Pluo in Zhejiang, CSPC of Hebei, Xinhua Pharmaceutical and Xinfa Pharmaceutical of Shandong rank well. Rank. chlorogold,tris(2,4-ditert-butylphenyl) phosphite supplier The vaccine also proved safe during the two-year trial, in which eleven doses were administered to randomly chosen patients with mild dementia. People who received the vaccine, known as AADvac1, experienced about the same numbers of side effects and adverse events as those who were given a placebo. chlorogold,tris(2,4-ditert-butylphenyl) phosphite vendor Cancer patients may have short bursts of high expenditures for treatments, while heart disease patients are often incurring a more chronic economic burden due to drug costs, procedures, clinician visits, and hospital stays,” a coauthor said in a statement.
Several authors disclosed financial ties to the pharmaceutical, medical device, medical technology, and health insurance industries.
Researchers say an extra dose of two-dose COVID-19 vaccines may improve immune system protection for organ transplant patients, a group that’s so far responded poorly to two-dose vaccines.
“Our findings suggest clinical trials are warranted to determine if transplant recipients should receive COVID-19 vaccine booster doses as standard clinical practice, similar to what is currently done with hepatitis B and influenza vaccinations for this population,” said study lead author Dr. William Werbel. He is an infectious diseases research fellow at the Johns Hopkins School of Medicine in Baltimore.
People who receive a heart, lung, kidney or other solid organ transplant often take drugs to suppress their immune system and prevent rejection, but those drugs can interfere with the body’s ability to make antibodies in response to vaccines.
In two previous studies, only 17% of transplant recipients produced sufficient antibodies after one shot of a two-dose COVID-19 vaccine, and only 54% produced sufficient antibodies after the second dose, researchers reported.
Even transplant recipients who produced antibodies had levels well below those typically seen in people with healthy immune systems, the findings showed.
In the new study, the researchers evaluated 30 transplant recipients who previously received two doses of either the Moderna or Pfizer/BioNTech vaccine. None had reported an illness or a positive test for SARS-CoV-2 prior to vaccination. All were taking multiple immunosuppressive medications to prevent organ rejection.
Between March 20 and May 10, all participants got a third dose of either one of the Moderna or Pfizer vaccines, or they got the Johnson & Johnson shot.
“A third of the participants who had negative antibody levels and all who had low positive [antibody] levels before the booster increased their immune response after a third vaccine dose,” said study senior author Dr. Dorry Segev. He directs the Epidemiology Research Group in Organ Transplantation at Hopkins.
A week after receiving their third dose, 23 patients completed a questionnaire and some reported generally mild or moderate side effects. One patient had severe arm pain and another reported a severe headache. No patients reported fever or an allergic reaction.
There was one case of mild organ rejection, according to the report published online June 15 in the Annals of Internal Medicine.
Segev said the reactions seem acceptable, given the benefits that vaccines can confer.
Meanwhile, Werbel urged transplant patients and other immunocompromised patients to be careful.
“Although the third vaccine dose appears to raise the immune response of transplant recipients to higher levels than after one or two doses, these people may still be at greater risk for SARS-CoV-2 infection than the general population who have been vaccinated,” he said in a Hopkins news release.
“Therefore, we recommend that transplant recipients and other immunocompromised people continue to wear masks, maintain physical distancing and practice other COVID-19 safety measures,” Werbel added.
Incyte Corporation (Nasdaq:INCY) announced today that the U.S. Food and Drug Administration (FDA) has extended the review period for the New Drug Application (NDA) for ruxolitinib cream for the treatment of atopic dermatitis (AD). The Prescription Drug User Fee Act (PDUFA) action date has been extended by three months to September 21, 2021.
The FDA extended the PDUFA action date to allow time to review additional analyses of previously submitted data provided by Incyte in response to the FDA’s information request. The submission of the additional information has been determined by the FDA to constitute a Major Amendment to the NDA, resulting in an extension of the PDUFA goal date.
“We are confident in the potential of ruxolitinib cream to offer a safe and effective treatment option for atopic dermatitis and will continue to work with the FDA to bring this targeted topical therapy to patients in the U.S. as soon as possible,” said Steven Stein, M.D., Chief Medical Officer, Incyte.
Ruxolitinib cream is a proprietary formulation of Incyte’s selective JAK1/JAK2 inhibitor ruxolitinib that has been designed for topical application. Ruxolitinib cream is currently in Phase 3 development for the treatment of adolescents and adults with atopic dermatitis (TRuE-AD) and vitiligo (TRuE-V). Incyte has worldwide rights for the development and commercialization of ruxolitinib cream.
Incyte is a Wilmington, Delaware-based, global biopharmaceutical company focused on finding solutions for serious unmet medical needs through the discovery, development and commercialization of proprietary therapeutics. For additional information on Incyte, please visit Incyte.com and follow @Incyte.
Except for the historical information set forth herein, the matters set forth in this press release, including statements regarding the Company’s ongoing clinical development program for ruxolitinib cream as well as its dermatology program generally, and whether and when ruxolitinib cream will be approved for use in the U.S. or elsewhere for atopic dermatitis or any other indication, contain predictions, estimates and other forward-looking statements.
These forward-looking statements are based on the Company’s current expectations and subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: unanticipated delays; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the effects of the COVID-19 pandemic and measures to address the pandemic on the Company’s clinical trials, supply chain, other third-party providers and development and discovery operations; determinations made by the FDA; the efficacy or safety of the Company’s products; the acceptance of the Company’s products in the marketplace; market competition; sales, marketing, manufacturing and distribution requirements; and other risks detailed from time to time in the Company’s reports filed with the Securities and Exchange Commission, including its annual report and its quarterly report on Form 10-Q for the quarter ended March 31, 2021. The Company disclaims any intent or obligation to update these forward-looking statements.
A polished, perfectly put-together face can be a huge boost to your confidence, but a new study shows that many of the cosmetics that help achieve that look might also be harmful to your health.
In the United States and Canada, plenty of beauty products appear to contain high levels of per- and polyfluoroalkyl substances (PFAS), a potentially toxic class of chemicals linked to some serious health conditions, researchers report.
In particular, most waterproof mascaras, liquid lipsticks and foundations contained high levels of fluorine, an indicator that PFAS are in the product.
“Those three categories were more likely than others to have high fluorine,” said researcher Tom Bruton, a senior scientist at the Green Science Policy Institute, an independent research and environmental advocacy organization in Berkeley, Calif.
The chemicals are used to make cosmetics more durable, and to make them spread better, Bruton explained. No company names were listed in the report, though the researchers said the problem is “widespread. chlorogold,tris(2,4-ditert-butylphenyl) phosphite factory At present, the additional 300 tons of sunniamine, 300 tons of fluoxamic acid, and 200 tons of cyprodinil have been successfully put into production, and the profitability is good.
Several authors disclosed financial ties to the pharmaceutical, medical device, medical technology, and health insurance industries.
Researchers say an extra dose of two-dose COVID-19 vaccines may improve immune system protection for organ transplant patients, a group that’s so far responded poorly to two-dose vaccines.
“Our findings suggest clinical trials are warranted to determine if transplant recipients should receive COVID-19 vaccine booster doses as standard clinical practice, similar to what is currently done with hepatitis B and influenza vaccinations for this population,” said study lead author Dr. William Werbel. He is an infectious diseases research fellow at the Johns Hopkins School of Medicine in Baltimore.
People who receive a heart, lung, kidney or other solid organ transplant often take drugs to suppress their immune system and prevent rejection, but those drugs can interfere with the body’s ability to make antibodies in response to vaccines.
In two previous studies, only 17% of transplant recipients produced sufficient antibodies after one shot of a two-dose COVID-19 vaccine, and only 54% produced sufficient antibodies after the second dose, researchers reported.
Even transplant recipients who produced antibodies had levels well below those typically seen in people with healthy immune systems, the findings showed.
In the new study, the researchers evaluated 30 transplant recipients who previously received two doses of either the Moderna or Pfizer/BioNTech vaccine. None had reported an illness or a positive test for SARS-CoV-2 prior to vaccination. All were taking multiple immunosuppressive medications to prevent organ rejection.
Between March 20 and May 10, all participants got a third dose of either one of the Moderna or Pfizer vaccines, or they got the Johnson & Johnson shot.
“A third of the participants who had negative antibody levels and all who had low positive [antibody] levels before the booster increased their immune response after a third vaccine dose,” said study senior author Dr. Dorry Segev. He directs the Epidemiology Research Group in Organ Transplantation at Hopkins.
A week after receiving their third dose, 23 patients completed a questionnaire and some reported generally mild or moderate side effects. One patient had severe arm pain and another reported a severe headache. No patients reported fever or an allergic reaction.
There was one case of mild organ rejection, according to the report published online June 15 in the Annals of Internal Medicine.
Segev said the reactions seem acceptable, given the benefits that vaccines can confer.
Meanwhile, Werbel urged transplant patients and other immunocompromised patients to be careful.
“Although the third vaccine dose appears to raise the immune response of transplant recipients to higher levels than after one or two doses, these people may still be at greater risk for SARS-CoV-2 infection than the general population who have been vaccinated,” he said in a Hopkins news release.
“Therefore, we recommend that transplant recipients and other immunocompromised people continue to wear masks, maintain physical distancing and practice other COVID-19 safety measures,” Werbel added.
Incyte Corporation (Nasdaq:INCY) announced today that the U.S. Food and Drug Administration (FDA) has extended the review period for the New Drug Application (NDA) for ruxolitinib cream for the treatment of atopic dermatitis (AD). The Prescription Drug User Fee Act (PDUFA) action date has been extended by three months to September 21, 2021.
The FDA extended the PDUFA action date to allow time to review additional analyses of previously submitted data provided by Incyte in response to the FDA’s information request. The submission of the additional information has been determined by the FDA to constitute a Major Amendment to the NDA, resulting in an extension of the PDUFA goal date.
“We are confident in the potential of ruxolitinib cream to offer a safe and effective treatment option for atopic dermatitis and will continue to work with the FDA to bring this targeted topical therapy to patients in the U.S. as soon as possible,” said Steven Stein, M.D., Chief Medical Officer, Incyte.
Ruxolitinib cream is a proprietary formulation of Incyte’s selective JAK1/JAK2 inhibitor ruxolitinib that has been designed for topical application. Ruxolitinib cream is currently in Phase 3 development for the treatment of adolescents and adults with atopic dermatitis (TRuE-AD) and vitiligo (TRuE-V). Incyte has worldwide rights for the development and commercialization of ruxolitinib cream.
Incyte is a Wilmington, Delaware-based, global biopharmaceutical company focused on finding solutions for serious unmet medical needs through the discovery, development and commercialization of proprietary therapeutics. For additional information on Incyte, please visit Incyte.com and follow @Incyte.
Except for the historical information set forth herein, the matters set forth in this press release, including statements regarding the Company’s ongoing clinical development program for ruxolitinib cream as well as its dermatology program generally, and whether and when ruxolitinib cream will be approved for use in the U.S. or elsewhere for atopic dermatitis or any other indication, contain predictions, estimates and other forward-looking statements.
These forward-looking statements are based on the Company’s current expectations and subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: unanticipated delays; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the effects of the COVID-19 pandemic and measures to address the pandemic on the Company’s clinical trials, supply chain, other third-party providers and development and discovery operations; determinations made by the FDA; the efficacy or safety of the Company’s products; the acceptance of the Company’s products in the marketplace; market competition; sales, marketing, manufacturing and distribution requirements; and other risks detailed from time to time in the Company’s reports filed with the Securities and Exchange Commission, including its annual report and its quarterly report on Form 10-Q for the quarter ended March 31, 2021. The Company disclaims any intent or obligation to update these forward-looking statements.
A polished, perfectly put-together face can be a huge boost to your confidence, but a new study shows that many of the cosmetics that help achieve that look might also be harmful to your health.
In the United States and Canada, plenty of beauty products appear to contain high levels of per- and polyfluoroalkyl substances (PFAS), a potentially toxic class of chemicals linked to some serious health conditions, researchers report.
In particular, most waterproof mascaras, liquid lipsticks and foundations contained high levels of fluorine, an indicator that PFAS are in the product.
“Those three categories were more likely than others to have high fluorine,” said researcher Tom Bruton, a senior scientist at the Green Science Policy Institute, an independent research and environmental advocacy organization in Berkeley, Calif.
The chemicals are used to make cosmetics more durable, and to make them spread better, Bruton explained. No company names were listed in the report, though the researchers said the problem is “widespread. chlorogold,tris(2,4-ditert-butylphenyl) phosphite factory At present, the additional 300 tons of sunniamine, 300 tons of fluoxamic acid, and 200 tons of cyprodinil have been successfully put into production, and the profitability is good.

