We serve Chemical Name:NSC 95397 CAS:93718-83-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
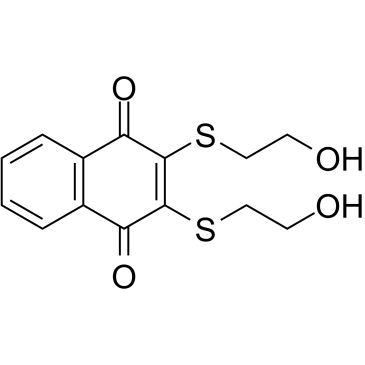
Chemical Name:NSC 95397
CAS.NO:93718-83-3
Synonyms:2,3-bis(2-hydroxyethylsulfanyl)naphthalene-1,4-dione
Molecular Formula:C14H14O4S2
Molecular Weight:310.39
HS Code:2930909090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:499.2ºC at 760mmHg
Density:1.46g/cm3
Index of Refraction:
PSA:125.20000
Exact Mass:310.03300
LogP:1.72820
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like 2,3-bis(2-hydroxyethylsulfanyl)naphthalene-1,4-dione chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,3-bis(2-hydroxyethylsulfanyl)naphthalene-1,4-dione physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,3-bis(2-hydroxyethylsulfanyl)naphthalene-1,4-dione Use and application,2,3-bis(2-hydroxyethylsulfanyl)naphthalene-1,4-dione technical grade,usp/ep/jp grade.
Related News: Though Innova previously applied for a regulatory green light, the test has not been authorized or approved by the FDA for use in the U.S.—however, during inspections of the company’s California facilities in March and April, FDA investigators said they found the test was already being sold and distributed. NSC 95397 manufacturer Amanpour is based in London, and said she feels fortunate to have health insurance through work and incredible doctors who are treating me in a country underpinned by, of course, the brilliant NHS [National Health Service],” CNN reported.
“I want to applaud Christiane Amanpour for her candor, bravery and always working towards the greater good,” he said in a statement. “As a cancer survivor, I too encourage people to listen to their bodies and get all early cancer screenings available to them. NSC 95397 supplier At present, China’s bulk drug industry is mainly focused on bulk drug substances, and there is huge room for development in terms of specialty drug substances and patented drug substances. NSC 95397 vendor Amanpour is based in London, and said she feels fortunate to have health insurance through work and incredible doctors who are treating me in a country underpinned by, of course, the brilliant NHS [National Health Service],” CNN reported.
“I want to applaud Christiane Amanpour for her candor, bravery and always working towards the greater good,” he said in a statement. “As a cancer survivor, I too encourage people to listen to their bodies and get all early cancer screenings available to them. NSC 95397 factory Though Innova previously applied for a regulatory green light, the test has not been authorized or approved by the FDA for use in the U.S.—however, during inspections of the company’s California facilities in March and April, FDA investigators said they found the test was already being sold and distributed.
“I want to applaud Christiane Amanpour for her candor, bravery and always working towards the greater good,” he said in a statement. “As a cancer survivor, I too encourage people to listen to their bodies and get all early cancer screenings available to them. NSC 95397 supplier At present, China’s bulk drug industry is mainly focused on bulk drug substances, and there is huge room for development in terms of specialty drug substances and patented drug substances. NSC 95397 vendor Amanpour is based in London, and said she feels fortunate to have health insurance through work and incredible doctors who are treating me in a country underpinned by, of course, the brilliant NHS [National Health Service],” CNN reported.
“I want to applaud Christiane Amanpour for her candor, bravery and always working towards the greater good,” he said in a statement. “As a cancer survivor, I too encourage people to listen to their bodies and get all early cancer screenings available to them. NSC 95397 factory Though Innova previously applied for a regulatory green light, the test has not been authorized or approved by the FDA for use in the U.S.—however, during inspections of the company’s California facilities in March and April, FDA investigators said they found the test was already being sold and distributed.

