We serve Chemical Name:4-(4-ethylsulfonyl-2-nitrophenyl)morpholine CAS:942474-41-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
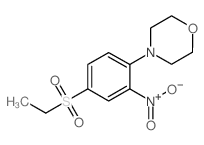
Chemical Name:4-(4-ethylsulfonyl-2-nitrophenyl)morpholine
CAS.NO:942474-41-1
Synonyms:or8359
Molecular Formula:C12H16N2O5S
Molecular Weight:300.33100
HS Code:2934999090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:100.81000
Exact Mass:300.07800
LogP:2.89400
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like or8359 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,or8359 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,or8359 Use and application,or8359 technical grade,usp/ep/jp grade.
Related News: After testing, our quality assurance staff confirms that everything has been performed correctly in accordance with GMP from manufacturing of API to quality testing. 4-(4-ethylsulfonyl-2-nitrophenyl)morpholine manufacturer In March 2021, Reuters reported that a former Lilly human resources officer, Amrit Mula, had identified internally some of the same violations later documented by the FDA. Mula was forced out of the company in early 2019 after Lilly executives sought to downplay her findings, according to a letter demanding compensation for damages that her attorneys sent to the company. 4-(4-ethylsulfonyl-2-nitrophenyl)morpholine supplier On April 8, a group of employees filed an anonymous complaint internally alleging that an executive at its Branchburg, New Jersey, factory had altered documents required by the U.S. Food and Drug Administration. 4-(4-ethylsulfonyl-2-nitrophenyl)morpholine vendor At present, Teva can produce more than 300 generic drugs, and the API department has approximately 650 authorized patents and patent applications worldwide. It is also the generic drug company with the most challenges in patenting ParagraphIV in the world. 4-(4-ethylsulfonyl-2-nitrophenyl)morpholine factory After testing, our quality assurance staff confirms that everything has been performed correctly in accordance with GMP from manufacturing of API to quality testing.

