We serve Chemical Name:7-methoxy-1,4-dihydro-1,6-naphthyridin-4-one CAS:952138-18-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
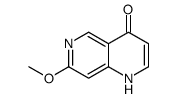
Chemical Name:7-methoxy-1,4-dihydro-1,6-naphthyridin-4-one
CAS.NO:952138-18-0
Synonyms:2-Methoxy-5H-pyrido(3′,2′:5,6)(1,4)thiazino(2,3-b)quinoxaline;7-Methoxy-1,4,6-benzo[b]triazaphenothiazine;7-methoxy-1,4-dihydro-1,6-naphthyridine-4-one;7-methoxy-1H-1,6-naphthyridin-4-one
Molecular Formula:C9H8N2O2
Molecular Weight:176.17200
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:54.98000
Exact Mass:176.05900
LogP:0.93170
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-Methoxy-5H-pyrido(3′,2′:5,6)(1,4)thiazino(2,3-b)quinoxaline chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,7-methoxy-1H-1,6-naphthyridin-4-one physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Methoxy-5H-pyrido(3′,2′:5,6)(1,4)thiazino(2,3-b)quinoxaline Use and application,7-methoxy-1,4-dihydro-1,6-naphthyridine-4-one technical grade,usp/ep/jp grade.
Related News: Bulk bulk medicines: overcapacity, low prices, future capacity and output will be reduced, and it is possible to transfer foreign production. 7-methoxy-1,4-dihydro-1,6-naphthyridin-4-one manufacturer Bulk bulk medicines: overcapacity, low prices, future capacity and output will be reduced, and it is possible to transfer foreign production. 7-methoxy-1,4-dihydro-1,6-naphthyridin-4-one supplier TSA notified airlines Saturday about the restrictions. CNN is reaching out to TSA for comment. 7-methoxy-1,4-dihydro-1,6-naphthyridin-4-one vendor Drug manufacturers make medicines by mixing APIs and pharmaceutical excipients. 7-methoxy-1,4-dihydro-1,6-naphthyridin-4-one factory On April 8, a group of employees filed an anonymous complaint internally alleging that an executive at its Branchburg, New Jersey, factory had altered documents required by the U.S. Food and Drug Administration.

