We serve Chemical Name:tributylgermane CAS:998-39-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
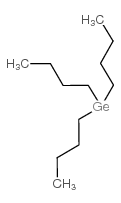
Chemical Name:tributylgermane
CAS.NO:998-39-0
Synonyms:MFCD00077987;Tri-n-butylgermanium hydride;Germane,tributyl;tributylgermanium hydride
Molecular Formula:C12H28Ge
Molecular Weight:244.99100
HS Code:2931900090
Physical and Chemical Properties:
Melting point:<0ºC
Boiling point:123 °C20 mm Hg(lit.)
Density:0.949 g/mL at 25 °C(lit.)
Index of Refraction:n20/D 1.45(lit.)
PSA:
Exact Mass:246.14000
LogP:4.99400
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like MFCD00077987 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,tributylgermanium hydride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Germane,tributyl Use and application,tributylgermanium hydride technical grade,usp/ep/jp grade.
Related News: Government prosecutors often consider whether a corporation made a genuine effort to investigate and address potentially illegal activities once learning of them. tributylgermane manufacturer A number of UK citizens who failed to leave the coronavirus-struck Chinese city of Wuhan last week are now on a second flight to Europe, UK Foreign Secretary Dominic Raab said Sunday. tributylgermane supplier The company has now selected a single engineered iPSC clone, and generated and fully-characterized the master engineered iPSC bank for GMP production of FT819. tributylgermane vendor As Reuters reported last month, Lilly tapped the Washington D.C. law firm Covington & Burling LLP to investigate the alleged alterations, which the employees said were meant to downplay serious quality control problems at the plant producing the drugmaker’s COVID-19 antibody treatment. tributylgermane factory As Reuters reported last month, Lilly tapped the Washington D.C. law firm Covington & Burling LLP to investigate the alleged alterations, which the employees said were meant to downplay serious quality control problems at the plant producing the drugmaker’s COVID-19 antibody treatment.

