We serve Chemical Name:(3,4-dimethyl-1,2-oxazol-5-yl)urea CAS:99980-24-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
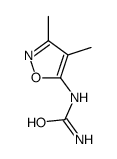
Chemical Name:(3,4-dimethyl-1,2-oxazol-5-yl)urea
CAS.NO:99980-24-2
Synonyms:(3,4-dimethyl-1,2-oxazol-5-yl)urea
Molecular Formula:C6H9N3O2
Molecular Weight:155.15500
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:82.14000
Exact Mass:155.06900
LogP:1.36880
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (3,4-dimethyl-1,2-oxazol-5-yl)urea chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(3,4-dimethyl-1,2-oxazol-5-yl)urea physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(3,4-dimethyl-1,2-oxazol-5-yl)urea Use and application,(3,4-dimethyl-1,2-oxazol-5-yl)urea technical grade,usp/ep/jp grade.
Related News: The CRL for the Cabenuva injection, containing two active ingredients cabotegravir and Janssen��s rilpivirine, follows U.S. market approval in April for its once-a-day pill Dovato, also a two-drug combination. (3,4-dimethyl-1,2-oxazol-5-yl)urea manufacturer That impacts airlines’ business, making it less financially attractive for them to fly those routes to China. (3,4-dimethyl-1,2-oxazol-5-yl)urea supplier Vietnam recently barred almost all flights to and from mainland China, Hong Kong and Macau until May 1, according to the United States Federal Aviation Administration. (3,4-dimethyl-1,2-oxazol-5-yl)urea vendor That impacts airlines’ business, making it less financially attractive for them to fly those routes to China. (3,4-dimethyl-1,2-oxazol-5-yl)urea factory As a result, the Company��s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy.

