We serve Chloral hydrate CAS:302-17-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
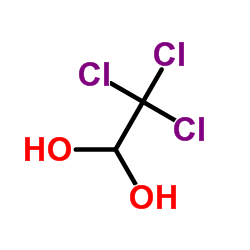
Contact us for information like Chloral hydrate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1,1-dihydroxy-2,2,2-trichloroethane physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Trichloroacetaldehyde monohydrate Use and application,2,2,2-trichloro-1,1-ethanediol technical grade,usp/ep/jp grade.
Related News: The mode of cooperation between domestic API companies and large international companies can lower the export threshold.2-(2-bromoethoxy)-1,3,5-trichlorobenzene manufacturer The risk-based score generated by the tool helps sponsors gain advance understanding of the appropriate level of clinical supply management oversight needed to help get their project started on time and maintain momentum as the study progresses.2-Methyl-3-nitrobenzotrifluoride supplier The news comes after China’s National Health Commission issued a notice on the treatment of the coronavirus on Monday, asking medical institutions to “actively promote the role of traditional Chinese medicine (TCM) during treatment.”3-Amino-2-bromo-4-picoline vendor The company’s iPSC product platform unites stem cell biology and precision genetic engineering to create renewable master engineered iPSC lines that can be repeatedly used to mass produce cancer-fighting immune cells, replacing the high production costs, weeks of manufacturing time, and complex engineering processes required for current-generation CAR T-cell immunotherapies with an off-the-shelf product that has the potential to reach many more patients.The company’s iPSC product platform unites stem cell biology and precision genetic engineering to create renewable master engineered iPSC lines that can be repeatedly used to mass produce cancer-fighting immune cells, replacing the high production costs, weeks of manufacturing time, and complex engineering processes required for current-generation CAR T-cell immunotherapies with an off-the-shelf product that has the potential to reach many more patients.

