We serve Chlorodifluoroacetic acid CAS:76-04-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
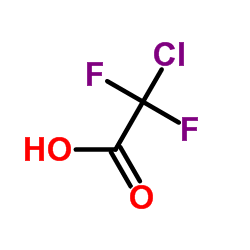
Contact us for information like Chlorodifluoroacetic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-chloro-2,2-difluoroacetic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Chlorodifluoroacetic acid Use and application,2-chloro-2,2-difluoroacetic acid technical grade,usp/ep/jp grade.
Related News: GlaxoSmithKline’s HIV drugs division ViiV Healthcare said on Saturday that the U.S. Food and Drug Administration declined to approve its long acting HIV injection.3,5-Bis-phenylazo-4-methoxy-toluol manufacturer The World Health Organisation (WHO) describes coronaviruses as a large family of viruses ranging from the common cold to more severe diseases such as Severe Acute Respiratory Syndrome (SARS), which killed nearly 800 people globally during a 2002/03 outbreak that also started in China.Hexanoic acid, 2-cyano-2-(3-methylbutyl)-5-oxo-, ethyl ester supplier Human iPSCs possess the unique dual properties of unlimited self-renewal and differentiation potential into all cell types of the body.3,3′-Dichlor-5,5′-dijodsalicyl vendor Russia, which had temporarily stopped issuing work visas to Chinese citizens, is also halting visa-free entry for Chinese tour groups, the government said. Moscow has also stopped issuing electronic tourist visas to individual Chinese travelers.Beneficial drug raw materials refer to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs). They are mainly used to meet the needs of original multinational pharmaceutical companies and emerging biopharmaceutical companies for innovative drugs in clinical drug research, registration approval and commercialization sales , Which also contains advanced intermediates used in the manufacture of the drug substance that need to be regulated by regulatory authorities.

