We serve Collagen hydrolyzates CAS:92113-31-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
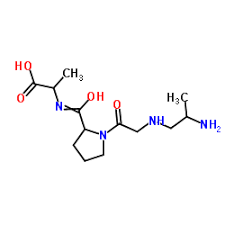
Contact us for information like Collagen hydrolyzates chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Hydrolyzed collagen physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Collagen hydrolyzates Use and application,Collagen hydrolyzates technical grade,usp/ep/jp grade.
Related News: There are no reliable vaccines available to rid your body of a coronavirus.2-FLUORO-5-METHYLBENZOIC ACID manufacturer Massachusetts state health officials, in conjunction with Massport, local health departments, and other medical partners, have responded to prevent the spread of the virus.2,3,4-Trifluorophenol supplier The foundational patent, which expires in 2034, is owned by MSK and is licensed exclusively to Fate Therapeutics for all human therapeutic uses.8-Bromo-1-octene vendor Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).Profitability continued to improve. The company’s comprehensive gross profit margin for the first three quarters was 36.06%, a year-on-year increase of 3.44 percentage points. Since this year, the company’s profitability has remained at a relatively high level. On the one hand, due to the decline in the trading business with lower profitability, the proportion has declined.

