We serve D-Valine CAS:640-68-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
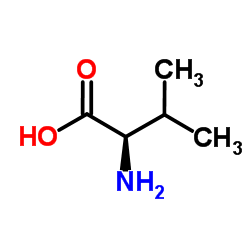
D-Valine
CAS RN: 640-68-6
| Solubility in 3M-HCl | Colorless & clear (c=5) |
| Specific rotation [α]D20 | -26.5°~-29.0°(c= 8, 6M-HCl) |
| Loss on drying | Not more than 0.3% |
| Residue on ignition (as sulfate) | Not more than 0.1% |
| Chloride (Cl) | Not more than 0.02% |
| Sulfate (SO4) | Not more than 0.03% |
| Heavy metals (as Pb) | Not more than 10ppm |
| Iron (Fe) | Not more than 10ppm |
| Ammonium (NH4) | Not more than 0.02% |
| Arsenic (As2O3) | Not more than 1ppm |
| Other amino acids | Not detected by T.L.C.(The spotted amount, 20μg) |
| Assay | 98.5%~101.0% |
| L-Isomer | Not more than 0.5% |
Contact us for information like D-Valine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(2R)-2-amino-3-methylbutanoic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,D-Valine Use and application,(2R)-2-amino-3-methylbutanoic acid technical grade,usp/ep/jp grade.
Related News: China has previously dealt with several bird flu outbreaks, the most recent in April 2019. In the new case, the H5N1 bird flu virus was found at a farm in the city of Shaoyang. The farm had 7,850 chickens, and more than half have died from the bird flu, the ministry said. It called the strain “highly pathogenic.”Methyl nicotinate manufacturer For example, an active ingredient to relieve pain is included in a painkiller.Acetamidine Hydrochloride supplier Optimize the reaction and improve the production process to reduce the production cost. At the same time, strengthen the determination and analysis of impurities to improve product quality.5,6,7,7a-tetrahydro-4H-thieno[3,2-c]pyridin-2-one,4-methylbenzenesulfonic acid vendor Green Valley said it would launch the drug “very soon” in China. The company also aims to roll out a phase-3 clinical trial with sites in the United States, Europe and Asia in early 2020 to facilitate global regulatory approval of the drug.Green Valley said it would launch the drug “very soon” in China. The company also aims to roll out a phase-3 clinical trial with sites in the United States, Europe and Asia in early 2020 to facilitate global regulatory approval of the drug.

