We serve Desmopressin Acetate CAS:62288-83-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
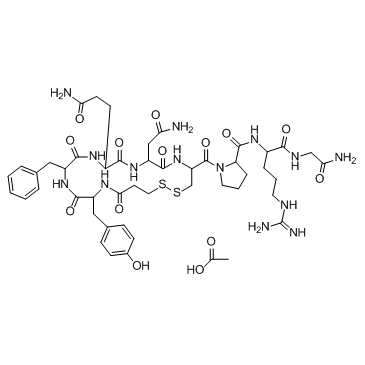
Contact us for information like Desmopressin Acetate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Desmopressin Acetate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Desmopressin Acetate Use and application,Desmopressin Acetate technical grade,usp/ep/jp grade.
Related News: It also said it would bar South Koreans from visiting China as tourists.3-Heptylacrolein manufacturer If an API is not ultrapure, a medicine cannot meet the strict quality criteria so the quality of an API plays a very important role.2-Bromo-1-(4-methoxyphenyl)ethanone supplier However, in 2018, the World Health Organization (WHO) gave its long-awaited nod to include TCM in its influential book classifying thousands of diseases — its first-ever official endorsement of the ancient practice.2-(2-amino-1,3-thiazol-4-yl)acetic acid vendor Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.The upstream industry for the R & D, production and sales of pharmaceutical intermediates is the basic chemical raw material industry, and the downstream industry is the chemical bulk drug and chemical pharmaceutical preparation industry.

