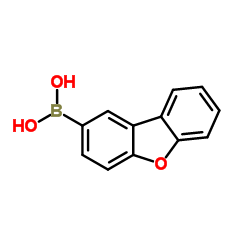
We serve dibenzofuran-2-ylboronic acid CAS:402936-15-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
Contact us for information like dibenzofuran-2-ylboronic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Dibenzo[b,d]furan-2-ylboronic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Dibenzofuranboronic acid Use and application,2-Dibenzofuranboronic acid technical grade,usp/ep/jp grade.
Related News: Taking Minuo Huawei as an example, in 2016, the capacity utilization rate and production-sales ratio of its multiple drug substance varieties reached more than 90%. Therefore, domestic API companies have expanded their production capacity in recent years to meet the growing demand for APIs.5-Bromo-2-chlorobenzotrifluoride manufacturer “We believe the iLet Bionics Pancreas System represents a true breakthrough therapy for the management of glycemia, particularly in type 1 diabetes,” said Ed Damiano, President and CEO of Beta Bionics. “We are partuclarly excited by the possibility that the iLet may be able to provide safer and more effective therapy in far more people than current therapies due to its simplicity of use.”2-Ethyl-3-methylpyrazine supplier During the deadly SARS pandemic of the early 2000s, another traditional Chinese herbal medicine, Banlangen, made from the root of a flowering woad plant and used to treat the common cold, was sold out in many pharmacies due to a popular but clinically unproven belief that it could help prevent SARS, which is closely related to the Wuhan coronavirus.Phenylacetic acid ethyl ester vendor At the same time, through years of imitation and advanced technology learning, more and more domestic companies have participated in the field of highly original and characteristic APIs.As a result, the Company’s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy.

