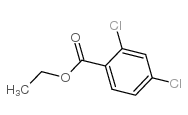
We serve ETHYL 2,4-DICHLOROBENZOATE CAS:56882-52-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
Contact us for information like ETHYL 2,4-DICHLOROBENZOATE chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,4-dichlorobenzoic acid ethyl ester physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,4-dichlorobenzoic acid ethyl ester Use and application,ETHYL 2,4-DICHLOROBENZOATE technical grade,usp/ep/jp grade.
Related News: Rigosertib is a small molecule that inhibits cellular signaling in cancer cells by acting as a RAS mimetic. Current clinical development of rigosertib is centered upon the therapeutic management of MDS, a heterogeneous group of bone marrow disorders characterized by ineffective hematopoiesis that often develop into acute myeloid leukaemia (AML).4,4'-Dibromo-4''-phenyltriphenylamine manufacturer Rigosertib is a small molecule that inhibits cellular signaling in cancer cells by acting as a RAS mimetic. Current clinical development of rigosertib is centered upon the therapeutic management of MDS, a heterogeneous group of bone marrow disorders characterized by ineffective hematopoiesis that often develop into acute myeloid leukaemia (AML).Iminodibenzyl supplier However, pharmaceutical intermediates are subdivided into primary intermediates and advanced intermediates. Because primary intermediate suppliers can only provide simple intermediate production, they are at the front end of the industrial chain. The pressure of competition and price is the greatest. The price fluctuations of basic chemical raw materials have a greater impact on them.1,1,3,3-Tetramethyl-1,3-Divinyldisilazane vendor With nearly 194 billion US dollars of original research medicines facing the expiration of patents in the past five years, more and more domestic companies are focusing on the corresponding characteristic APIs, and have started research and development and production preparations in advance. The scale of production and export of APIs will continue to expand and grow.With nearly 194 billion US dollars of original research medicines facing the expiration of patents in the past five years, more and more domestic companies are focusing on the corresponding characteristic APIs, and have started research and development and production preparations in advance. The scale of production and export of APIs will continue to expand and grow.

