We serve Ethyl 3-(isopropylamino)propanoate CAS:16217-22-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
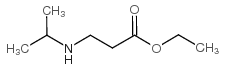
Contact us for information like Ethyl 3-(isopropylamino)propanoate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,ethyl 3-(propan-2-ylamino)propanoate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Ethyl 3-(isopropylamino)propanoate Use and application,Ethyl 3-(isopropylamino)propanoate technical grade,usp/ep/jp grade.
Related News: The fine chemical industry includes fine chemicals and special chemicals. Fine chemical products have the characteristics of a small amount of production, relatively specific application fields, and long and complex industrial chains. They are mainly used in medicine, pesticides, dyes, liquid crystals and other fields.2-Isopropylthioxanthone manufacturer They have also voiced frustrations about patients from mainland China hiding their travel and medical history, potentially endangering other patients.Magnesium (R)-3-hydroxybutanoate supplier Rigosertib, in its intravenous formulation, is currently in Phase 3 clinical development for the treatment of higher-risk MDS.3,4-Dimethylaniline vendor Rigosertib, in its intravenous formulation, is currently in Phase 3 clinical development for the treatment of higher-risk MDS.But they’ll be subject to up to 14 days of mandatory quarantine once they’re back in the US.

