We serve ethyl 5-(ethoxycarbonylsulfamoyl)-1-methylpyrazole-4-carboxylate CAS:159709-60-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
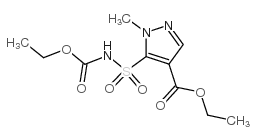
Contact us for information like ethyl 5-(ethoxycarbonylsulfamoyl)-1-methylpyrazole-4-carboxylate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,ethyl 5-(ethoxycarbonylsulfamoyl)-1-methylpyrazole-4-carboxylate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,ethyl 5-(ethoxycarbonylsulfamoyl)-1-methylpyrazole-4-carboxylate Use and application,ethyl 5-(ethoxycarbonylsulfamoyl)-1-methylpyrazole-4-carboxylate technical grade,usp/ep/jp grade.
Related News: On the other hand, the company has many new products in recent years, including Duqiao Base, Yancheng United Chemical and Dezhou United Chemical. These new products are mostly customized products with strong profitability, which has improved the gross profit margin of the industrial business.2-Methoxy-3-isobutyl pyrazine manufacturer At the same time, due to the increase in production costs and environmental protection cost pressures in Europe and the United States, as well as the improvement of process technology, production quality, and registration and certification capabilities of China’s bulk drug manufacturers, bulk drug companies have accelerated their transfer to China, and the bulk drug industry in China has produced Scale continues to increase.2-Amino-3-bromo-5-methylpyridine supplier With the death toll surging past 300 and 14,300 cases confirmed, authorities across the country have activated the highest public health emergency response, stepping up screening of arrivals from Wuhan.4-tert-Butylphenyl isothiocyanate vendor DMF is the main management method for APIs in developed countries in Europe and the United States. Under the DMF system, API companies can submit DMF filing documents to the regulatory authority at any time, but the regulatory authority will not conduct technical reviews on them. When the drug is administered, the regulatory authority will associate and review the drug substance and the preparation.INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.

