We serve exo-3,6-Methylene-1,2,3,6-tetrahydrophthalic anhydride CAS:2746-19-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
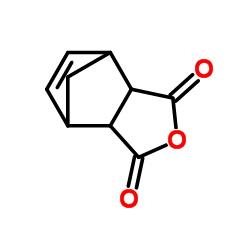
Contact us for information like exo-3,6-Methylene-1,2,3,6-tetrahydrophthalic anhydride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,cis-5-Norbornene-exo-2,3-dicarboxylic Anhydride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,cis-5-Norbornene-exo-2,3-dicarboxylic Anhydride Use and application,exo-3,6-Methylene-1,2,3,6-tetrahydrophthalic anhydride technical grade,usp/ep/jp grade.
Related News: According to statistics from the Price Supervision and Competition Bureau of the National Development and Reform Commission, China can produce more than 1,500 kinds of bulk drugs, with a total output of one million tons and an export volume of more than 60%. It has become the world’s second largest drug substance after the United States. Country of manufacture and largest exporter.1,3-BIS(ISOCYANATOMETHYL)CYCLOHEXANE manufacturer But now, the metropolis of 11 million in Hubei province has become the face of a deadly coronavirus outbreak — a stigma the people of Wuhan increasingly find themselves unable to shake off.3-Ethyl-5-(2-Hydroxyethyl)-4-Methylthiazolium Bromide supplier There is fierce competition for bulk APIs and overcapacity. However, some high-profit, small-scale drugs still have a good market space.4-(4-bromophenyl)dibenzofuran vendor Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

