We serve Fmoc-D-Arg(pbf)-OH CAS:187618-60-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
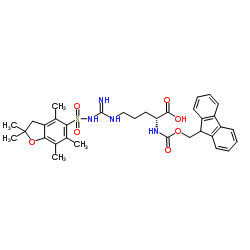
| Items of Analysis | Standard of Analysis | Test Results |
| Appearance | White powder | Conform |
| Purity (HPLC) | ≥98.0% | 99.31% |
| TLC | ≥98.0% | >98.0% |
| Optical Purity | ≤0.5% L-enantiomer | ND |
| Specific Rotation[a]20D | +4.5o±1.5o (C=1 in DMF) | +5.7o |
| Clarity of solution | 0.3 gram in 2ml DMF clear solution | Conform |
| Water | ≤2.0% | 0.16% |
| Loss on drying | ≤2.0% | 0.12% |
| Kaiser Test | <0.05% | <0.05% |
| Element analysis(C、H、N) | ≤5.0% | <5.0% |
| Conclusion | Conforms to Factory Standard | |
Contact us for information like Fmoc-D-Arg(pbf)-OH chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Nα-Fmoc-Nω-Pbf-D-arginine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Fmoc-D-Arg(pbf)-OH Use and application,Fmoc-D-Arg(Pbf)-OH technical grade,usp/ep/jp grade.
Related News: Coronaviruses (CoV) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV). A novel coronavirus (nCoV) is a new strain that has not been previously identified in humans.2,2-Dimethyl-4-(2,2,2-trifluoro-ethoxymethyl)-[1,3]dioxolane manufacturer Active pharmaceutical ingredients affect the structure and function of the human body. Inactive ingredients in a pharmaceutical may be referred to as “bulk pharmaceutical chemicals (BPCs).”N-((1H-imidazol-2-yl)methyl)-N-(4-(((pyridin-2-ylmethyl)amino)methyl)benzyl)-5,6,7,8-tetrahydroquinolin-5-amine supplier “Traditional medicine is a treasure of Chinese civilization embodying the wisdom of the nation and its people,” Xi said at the meeting in Beijing.1-propyl-3-methylimidazolium chloride vendor Inceptua Medicines Access is a business unit of the Inceptua Group. It offers full access solutions for the design, implementation and delivery of Pre-approval and Medicines Access Programs on behalf of biopharmaceutical companies.The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.

