We serve L-Serine benzyl ester hydrochloride CAS:60022-62-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
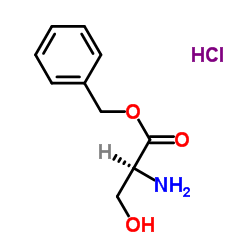
Chemical Name: L-Serine benzyl ester hydrochloride
CAS.NO: 60022-62-0
Molecular Formula:C10H14ClNO3
Molecular Weight: 231.67600
Synonyms:
benzyl (2S)-2-amino-3-hydroxypropanoate,hydrochloride
Physical and Chemical Properties:
Density:/
Boiling point: 360.4ºC at 760 mmHg
Melting point: 175 ° C
Flash point: 171.8ºC
Refractive index: -12 ° (C = 1, H2O)
Specification:
Appearance: White to light yellow crystal powder
Purity:≥98%
Moisture Content:< 0.1%
Impurity: < 0.1%
Packing:25 kg/drum, can also be packaged according to customer requirements
Storage:-20ºC℃ protected from light, cool and dry place, sealed and stored.
Application:Pharmaceutical Intermediate.
Contact us for information like L-Serine benzyl ester hydrochloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,benzyl (2S)-2-amino-3-hydroxypropanoate,hydrochloride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,benzyl (2S)-2-amino-3-hydroxypropanoate,hydrochloride Use and application,L-Serine benzyl ester hydrochloride technical grade,usp/ep/jp grade.
Related News: The news, however, was greeted with skepticism online in China, with many — including numerous medical experts — questioning whether the findings were supported by clinical evidence from treating coronavirus patients.S-methyl butanethioate manufacturer The term active pharmaceutical ingredient may refer to an active chemical within an FDA-regulated drug, or API might mean the entire drug with its active and inactive ingredients.4-Chloro-2,5-difluorobenzaldehyde supplier Refers to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs or innovative drugs). It mainly meets the needs of international original research drug companies and emerging biopharmaceutical companies for innovative drugs at various stages of clinical research, registration approval and commercialization of drugs. Contains advanced intermediates used in the manufacture of this drug substance that need to be regulated by regulatory authorities.5-fluorocytidine vendor Refers to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs or innovative drugs). It mainly meets the needs of international original research drug companies and emerging biopharmaceutical companies for innovative drugs at various stages of clinical research, registration approval and commercialization of drugs. Contains advanced intermediates used in the manufacture of this drug substance that need to be regulated by regulatory authorities.Cases recorded in Thailand, Taiwan, Germany, Vietnam, Japan, France and the United States involved patients who had not been to China.

