We serve L-Threonine CAS:72-19-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
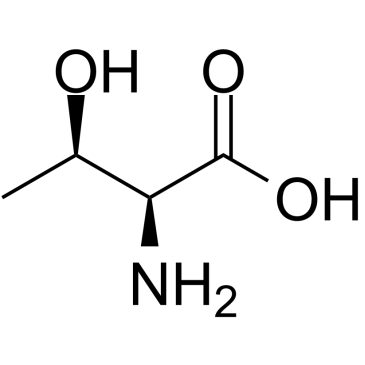
L-Threonine
An essential amino acid in human. It is widely used as an ingredient in infusion and infant formula.
| Cas.No | 72-19-5 |
| Assay | 99.0-101.0% |
| Specification | White crystals or crystalline powder, odorless or slightly characteristic odor, slightly sweet taste |
| Applications | Infusion |
| Packaging | 25kg&50kg |
| Pharmacopeia | JP,USP,EP,FCC |
| STORAGE | Controlled room temperature in tight container |
SPECIFICATION AND PROCEDURE
| State of solution (Transmittance) |
Not Less Than 98.0% |
| pH | 5.0~6.5 |
| Specific rotation[α]20D | -27.6~-29.0° |
| Specific rotation[α]25D | -26.7~-29.1° |
| Ammonium (NH4) | Not More Than 0.020% |
| Chloride (Cl) | Not More Than 0.020% |
| Sulfate (SO4) | Not More Than 0.020% |
| Iron (Fe) | Not More Than 10 ppm |
| Heavy metals (Pb)** | Not More Than 10 ppm |
| Arsenic (As2O3) | Not More Than 1 ppm |
| Loss on drying | Not More Than 0.20% |
| Residue on ignition | Not More Than 0.10% |
| Related substances | Not More Than 0.5% |
| Endotoxin* | Less Than 6.0 EU/g |
| Assay (dry basis) | 99.0~101.0% |
· * The endotoxin-certified grade will be supplied on request.
· ** FCC grade (Lead : Not More Than 5 mg/kg) will be supplied on request.
· This product meets requirements of residual solvents listed in the current JP, USP and EP.
Contact us for information like L-Threonine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,H-L-THR-OH physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,THR Use and application,L-THR technical grade,usp/ep/jp grade.
Related News: Especially tumor drugs with selective targeting characteristics. At present, about 80% of small drug (high-efficiency drug) APIs worldwide are patented drugs.4-(4-(Dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrile hydrobromide manufacturer The rigosertib Pre-approval Access Program is expected to launch in first half of 2020 and will allow Inceptua to supply intravenous rigosertib within designated countries, primarily and initially concentrated in selected countries in Europe, in response to physician requests for patients with higher-risk MDS who have exhausted all available treatment options, and are not eligible for or have no access to the INSPIRE study.2-methyl-2-(methylsulfanyl)propanaldoxime supplier Such was the apparent demand sparked by the notice that the compound formula sold out on some stores on China’s e-commerce platform Taobao.6-chloro-7H-purine vendor FT819 is derived from a clonal master engineered induced pluripotent stem cell (iPSC) line with complete elimination of T-cell receptor (TCR) expression and a novel 1XX CAR targeting CD19 inserted into the T-cell receptor alpha constant (TRAC) locus.FT819 is derived from a clonal master engineered induced pluripotent stem cell (iPSC) line with complete elimination of T-cell receptor (TCR) expression and a novel 1XX CAR targeting CD19 inserted into the T-cell receptor alpha constant (TRAC) locus.

